Subchondral cell density decreases significantly with increasing grade of degeneration in knee osteoarthritis.
Dr. Sathish Muthu, Department of Orthopaedics, Government Medical College, Karur, Tamil Nadu, India. E-mail: drsathishmuthu@gmail.com
Introduction: Subchondral milieu has been the region of interest in recent clinical trials demonstrating it as an effective treatment focus in knee osteoarthritis (OA). However, a systematic analysis of the subchondral cell population density is lacking in knee OA. Hence, this study aims to analyze the cell population density of the subchondral zone for every stage of cartilage degeneration to analyze the rationale behind this evolving treatment method for knee OA.
Materials and Methods: This is an ex vivo histopathological analysis of the distal femur and proximal tibia articular specimens from the patients undergoing total knee replacement for primary knee OA between November 2023 and March 2024. Two pathologists independently graded and analyzed the subchondral cell density based on the International Cartilage Repair Society cartilage injury grading system.
Results: We noted a significant association between the grade of cartilage degeneration and the subchondral cell density (r = 0.831, P < 0.001). We noted a statistically significant decrease in the cell density for every stage of cartilage injury compared to the control (P < 0.001). We also noted a significant decrease in cell density between the early and late stages of cartilage degeneration (P < 0.001). We did not note any significant difference in the cell density between the tibial and femoral articular cartilage for every grade of cartilage degeneration analyzed (P = 0.432).
Conclusion: Subchondral cell density decreases significantly with increasing grade of degeneration in knee OA. Subchondral milieu warrants attention to be considered as a potential treatment focus that could alter the disease progression in knee OA.
Keywords: Histopathology, knee osteoarthritis, subchondral zone, mesenchymal stromal cells, regenerative medicine.
Osteoarthritis (OA) of the knee is a progressive degenerative condition that significantly impacts joint biomechanics and quality of life, leading to disability and pain [1]. Despite being a pure cartilage disease, it rather affects various other regions of the joint from outer capsular synovium to subchondral regions. Hence, it could be called as a whole joint disease [2]. Bone marrow lesions are considered to be one of the hallmark characteristic features of OA that occurs before articular cartilage degeneration. This has led to a new stream of management methods targeting subchondral regions with growth factors and cell substitutes. In recent years, cellular therapy modalities utilizing autologous or allogeneic cultured mesenchymal stromal cells (MSCs) have emerged as a promising alternative in the landscape of regenerative therapies [3]. Utilization of bone marrow concentrates rich in MSCs, and growth factors have been considered one of the first-line treatments in the early OA targeting the bone marrow lesions and synovitis [4]. However, the best target to prevent the disease progress has not yet been identified [5]. In secondary osteonecrosis, sufficient MSCs have been noted in the intra-articular region in the synovial fluid of osteoarthritic knees [6]. However, the current treatment methods involving increasing the MSCs in the intra-articular region in the synovial fluid of the arthritic knees do not address the need [7]. Hence, alternative treatment methods focusing on subchondral regions of the knee have evolved. Recent clinical trials have demonstrated that implantation of MSCs in the subchondral bone of osteoarthritic knees is more effective in the long term in preventing total knee replacement compared to the intra-articular route [6, 8, 9]. However, the rationale behind the subchondral transplantation of MSCs lacks systematic analysis of the cell population density in knee OA. Subchondral bone is considered an integral component in the disease process [10]. Studies have noted that subchondral changes were not simply the result of the altered biomechanical loading in the degenerated joints; instead, they were considered to contribute to the cartilage injury in the early disease process [11]. Hence, this study aims to analyze the cell population density of the subchondral zone for every stage of cartilage degeneration to analyze the rationale behind this evolving treatment method for knee OA.
The study was conducted following approval of the protocol of conduct by the Institutional Ethical Committee (KMC/IEC/2023/11/3). This is an ex vivo histopathological analysis of the distal femur and proximal tibia articular specimens from the patients undergoing total knee replacement for primary knee OA between November 2023 and March 2024. We excluded samples from patients with comorbid illnesses that would affect the subchondral cell population including diabetes mellitus, chronic steroid intake, recent irradiation, and so on.
Sample preparation and grading
The saw-cut specimens from total knee replacement surgery were immediately transferred to formalin containers to aid in fixation and transported to the histopathology laboratory immediately for processing. The specimen was cut into blocks of size 1 cm3. We prepared four samples from different areas per grade of cartilage degeneration from the tibial and femoral side, respectively, for every patient included in the study. We also included four control samples from uninvolved articular areas with intact articular cartilage. Two pathologists independently graded and labeled the cartilage degeneration status in the articular surface of the block and labeled them accordingly. Any discrepancies in grading were resolved with discussion. We used the International Cartilage Repair Society cartilage injury grading system as shown in Fig. 1. Later, the blocks were embedded in a paraffin medium and sectioned using a microtome (HistoCore BIOCUT, Leica Biosysetms, Germany). The sections were then stained manually using hematoxylin and eosin stain for further analysis.
Cell density analysis
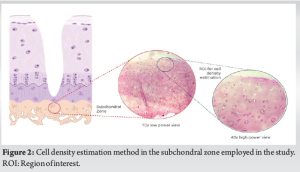
The stained slides were examined independently by two pathologists through a light microscope (LB 207, LaboMed Inc., USA) to estimate the number of cells in the subchondral region pertaining to one high-power field directly beneath the degenerated cartilage as shown in Fig. 2. Any discrepancy in cell counting was resolved with discussion.
Statistical analysis
We used mean and standard deviation to present continuous variables and percentage to present categorical variables. We included 4 samples from the femoral side (one for each grade of cartilage degeneration) and 4 samples from the tibial side (one for each grade of cartilage degeneration) resulting in 8 samples and 8 controls from every individual patient. We analyzed the correlation between the cell counts in the tibial and femoral subchondral zones and the grade of cartilage degeneration using Pearson’s correlation test. Differences between the different grades of cartilage degeneration were analyzed using one-way analysis of variance. A P < 0.05 was considered statistically significant. The statistical analysis was conducted using IBM SPSS Version 25 (Armonk, USA).
Characteristics of patients
We included the articular specimens from 21 patients who satisfied the inclusion criteria. The mean age of patients included in the study was 62.8 (±5.4) years. Of the 21 patients, 12 (57.1%) were female. The mean body mass index of the included patients was 25.5 (±3.4). We analyzed a total of 168 slides per grade of cartilage degeneration amounting to a total of 840 slides including control slides.
Cell density
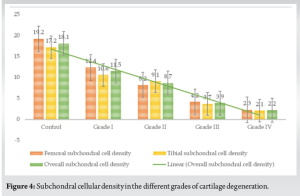
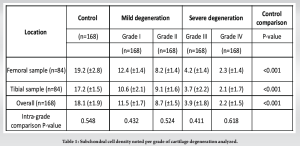
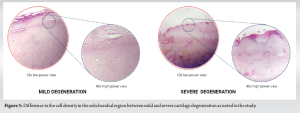
The outcome of cell density from the analysis is presented in Table 1. We noted a significant association between the grade of cartilage degeneration and the subchondral cell density (r = 0.831, P < 0.001). We noted a statistically significant decrease in the cell density for every stage of cartilage degeneration compared to the control (P < 0.001). We also noted a significant decrease between the early and late stages of cartilage degeneration (P < 0.001) as shown in Table 1 and Fig. 3. We did not note any significant difference in the cell density between the tibial and femoral articular cartilage for every grade of cartilage degeneration analyzed as shown in Fig. 4. We also did not note any gender differences in the cell density loss for every grade of cartilage degeneration analyzed (P = 0.583).
The integrity of the cartilage largely depends on the supporting subchondral bone and vice versa along with healthy synovial fluid and synovial covering [12]. The synovial fluid contains MSCs and their count increases with the ongoing disease process since they play a crucial role in maintaining joint homeostasis and repair [13]. They were considered to migrate from the synovial reservoir and therapies increasing the volume of MSCs involved in the repair of ongoing degenerated cartilage have been shown to provide pain relief and functional improvement in the short term [14, 15]. Although intra-articular injection has shown benefits in the short term, the long-term failures could be accounted to the limited adhesion of these progenitors to the degenerated cartilage considering the distinct variations between MSCs of bone marrow origin and synovial origin [14, 16]. Hence, alternative strategies were considered for this purpose. Of late subchondral milieu has been the region of interest for cellular transplantation considering the rationale that the subchondral progenitor population needs to be augmented to achieve chondral regeneration ameliorating the degenerative cascade. Results of recent clinical trials further strengthened this hypothesis thereby directing the focus of the research community to its favor. Our study adds to the literature by systematically analyzing the subchondral cellular density in every stage of cartilage degeneration to further strengthen the rationale of subchondral cellular augmentation as a management option that alters the natural history of knee OA. The key finding of our study is that a significant decrease in the subchondral cell density with increasing grade of cartilage degeneration which could be interpreted as reduced cellular density in the subchondral zone is a feature of the advancing disease process. Further, we also noted that not only the subchondral region of the femoral side is involved but also the tibial subchondral zone is equally involved and hence cellular augmentation is needed in both the sides of the joint to address the disease process effectively. We also noted the changes in the subchondral zone equally involved without any sexual preponderance. With the data that subchondral cellular loss accounts for the progressive degenerative disease process, transplantation of the MSCs in the subchondral milieu is advantageous in that it does not get washed by synovial fluid as in intra-articular delivery. Hernigou et al. [6, 17] in their randomized controlled trial established that the hypothesis works and has demonstrated subchondral transplantation of MSCs to reduce the incidence of total knee replacement from 4.6% noted in the intra-articular delivery to 1.3% per year at 15-year follow-up. They also noted that while only 20% (n = 12) of knees that received subchondral transplantation necessitated total knee replacement, 70% (n = 42) of knees that received intra-articular transplantation needed replacement surgery. Further, patients preferred knee with subchondral cell therapy to be more satisfactory. The possible explanation for the noted cellular changes in the subchondral zone in OA could be accounted for by the dynamic stress-bearing nature that complements the load-bearing of the joints [18, 19]. A sclerotic and acellular subcondral zone could result in transmission of increased load to the overlying cartilage resulting in increased secondary damage and degeneration [20, 21]. The vicious cycle of cartilage damage and bone stiffness results in OA [21]. Apart from the mechanical aspects, intricate biological regulatory interplay exists between the cartilage and the subchondral zone that contributes to the healthy and pathological nature of the overall joint making them a potential target for treatment [10, 22]. The study has some limitations. First, we analyzed the cellular density and did not profile the cell populations by immunohistochemistry to quantify the progenitor cell population. However, the supporting cells in the subchondral matrix along with the MSCs were accounted for in the current study. Individual variations in the subchondral cell population exist but we tried to overcome this variability by increasing the sample and patient numbers. Although we graded the subchondral foci based on the cartilage degeneration status, the duration of the disease process might have a role in the subchondral cellular population observed which was not taken into consideration in the interpretation of study findings. Future studies that account for the duration of the disease process along with detailed cellular profiling might enhance the understanding of the subchondral milieu in greater depth.
Subchondral cell density decreases significantly with increasing grade of degeneration in knee OA. Subchondral milieu warrants attention to be considered as a potential treatment focus that could alter the disease progression in knee OA.
- Subchondral cell density decreases in OA
- Subchondral cell density decreases between the early and late stages of cartilage degeneration
- No difference in the cell density is noted between the tibial and femoral articular cartilage.
References
- 1.Muthu S. Osteoarthritis, an old wine in a new bottle! World J Orthop 2023;14:1-5. [Google Scholar]
- 2.Yusup A, Kaneko H, Liu L, Ning L, Sadatsuki R, Hada S, et al. Bone marrow lesions, subchondral bone cysts and subchondral bone attrition are associated with histological synovitis in patients with end-stage knee osteoarthritis: A cross-sectional study. Osteoarthritis Cartilage 2015;23:1858-64. [Google Scholar]
- 3.Keeling LE, Belk JW, Kraeutler MJ, Kallner AC, Lindsay A, McCarty EC, et al. Bone marrow aspirate concentrate for the treatment of knee osteoarthritis: A systematic review. Am J Sports Med 2022;50:2315-23. [Google Scholar]
- 4.Chahla J, Piuzzi NS, Mitchell JJ, Dean CS, Pascual-Garrido C, LaPrade RF, et al. Intra-articular cellular therapy for osteoarthritis and focal cartilage defects of the knee: A systematic review of the literature and study quality analysis. J Bone Joint Surg Am 2016;98:1511-21. [Google Scholar]
- 5.Muthu S, Korpershoek J, Novais E, Tawy GF, Hollander AP, Martin I. Failure of cartilage regeneration: Emerging hypotheses and related therapeutic strategies. Nat Rev Rheumatol 2023;19:403-16. [Google Scholar]
- 6.Hernigou P, Auregan JC, Dubory A, Flouzat-Lachaniette CH, Chevallier N, Rouard H. Subchondral stem cell therapy versus contralateral total knee arthroplasty for osteoarthritis following secondary osteonecrosis of the knee. Int Orthop 2018;42:2563-71. [Google Scholar]
- 7.Ding JB, Hu K. Injectable therapies for knee osteoarthritis. Reumatologia 2021;59:330-9. [Google Scholar]
- 8.Carneiro DC, de Araújo LT, Santos GC, Damasceno PK, Vieira JL, Dos Santos RR, et al. Clinical trials with mesenchymal stem cell therapies for osteoarthritis: Challenges in the regeneration of articular cartilage. Int J Mol Sci 2023;24:9939. [Google Scholar]
- 9.Tjandra KC, Novriansyah R, Sudiasa IN, Ar A, Rahmawati NA, Dilogo IH. Modified mesenchymal stem cell, platelet-rich plasma, and hyaluronic acid intervention in early stage osteoarthritis: A systematic review, meta-analysis, and meta-regression of arthroscopic-guided intra-articular approaches. PLoS One 2024;19:e0295876. [Google Scholar]
- 10.Kasaeian A, Roemer FW, Ghotbi E, Ibad HA, He J, Wan M, et al. Subchondral bone in knee osteoarthritis: Bystander or treatment target? Skeletal Radiol 2023;52:2069-83. [Google Scholar]
- 11.Muratovic D, Findlay DM, Cicuttini FM, Wluka AE, Lee YR, Edwards S, et al. Bone marrow lesions in knee osteoarthritis: Regional differences in tibial subchondral bone microstructure and their association with cartilage degeneration. Osteoarthritis Cartilage 2019;27:1653-62. [Google Scholar]
- 12.Henrotin Y, Pesesse L, Sanchez C. Subchondral bone in osteoarthritis physiopathology: State-of-the art and perspectives. Biomed Mater Eng 2009;19:311-6. [Google Scholar]
- 13.Hügle T, Geurts J. What drives osteoarthritis?-synovial versus subchondral bone pathology. Rheumatology (Oxford) 2017;56:1461-71. [Google Scholar]
- 14.Sekiya I, Ojima M, Suzuki S, Yamaga M, Horie M, Koga H, et al. Human mesenchymal stem cells in synovial fluid increase in the knee with degenerated cartilage and osteoarthritis. J Orthop Res 2012;30:943-9. [Google Scholar]
- 15.Jones EA, Crawford A, English A, Henshaw K, Mundy J, Corscadden D, et al. Synovial fluid mesenchymal stem cells in health and early osteoarthritis: Detection and functional evaluation at the single-cell level. Arthritis Rheum 2008;58:1731-40. [Google Scholar]
- 16.Jeyaraman M, Muthu S, Jeyaraman N, Ranjan R, Jha SK, Mishra P. Synovium derived mesenchymal stromal cells (Sy-MSCs): A promising therapeutic paradigm in the management of knee osteoarthritis. Indian J Orthop 2022;56:1-15. [Google Scholar]
- 17.Hernigou P, Bouthors C, Bastard C, Flouzat Lachaniette CH, Rouard H, Dubory A. Subchondral bone or intra-articular injection of bone marrow concentrate mesenchymal stem cells in bilateral knee osteoarthritis: What better postpone knee arthroplasty at fifteen years? A randomized study. Int Orthop 2021;45:391-9. [Google Scholar]
- 18.Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci 2010;1192:230-7. [Google Scholar]
- 19.Neogi T, Nevitt M, Niu J, Sharma L, Roemer F, Guermazi A, et al. Subchondral bone attrition may be a reflection of compartment-specific mechanical load: The MOST study. Ann Rheum Dis 2010;69:841-4. [Google Scholar]
- 20.Pan J, Zhou X, Li W, Novotny JE, Doty SB, Wang L. In situ measurement of transport between subchondral bone and articular cartilage. J Orthop Res 2009;27:1347-52. [Google Scholar]
- 21.Li G, Yin J, Gao J, Cheng TS, Pavlos NJ, Zhang C, et al. Subchondral bone in osteoarthritis: Insight into risk factors and microstructural changes. Arthritis Res Ther 2013;15:223. [Google Scholar]
- 22.Hu Y, Chen X, Wang S, Jing Y, Su J. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res 2021;9:20. [Google Scholar]