This case report highlights the importance of the potential brittle design of trial components, which can – in the event of breakage – lead to dramatic complications (e.g., dislocation, wear, etc.)
Dr. Mathias van den Broek, Department of Orthopaedic Surgery, AZ Sint Blasius, Dendermonde, Belgium. E-mail: mathias.vandenbroek@azsintblasius.be
Introduction: Dislocation is one of the most common causes of patient and surgeon dissatisfaction following total hip arthroplasty (THA). In this case, we describe the presence of broken plastic particles, originating from the plastic trial femoral head (TFH) which caused total hip dislocation.
Case Report: A 67-year-old man with avascular necrosis of his left hip, who underwent a hybrid THA, dislocated his hip twice in the first 2 months after the initial surgery. A computed tomography scan revealed a well-oriented cup and stem and the presence of two dense intra-articular particles, later identified as the possible cause of dislocation. The particles were removed during revision surgery and were retrospectively determined to be broken TFH particles, made of hard plastic.
Conclusion: We believe that the brittle design of certain TFH components is vulnerable to damage during perioperative trialing and testing; therefore, care must be taken to check the integrity of these devices during hip surgery. It is important to recognize the existence of such a rare complication.
Keywords: Total hip arthroplasty, hip joint, joint dislocations.
Dislocation following total hip arthroplasty (THA) is one of the most common causes of patient and surgeon dissatisfaction [1, 2]. Most dislocations (60–70%) occur within the first 6 weeks after surgery and up to one third become recurrent. Several patient-, surgeon-, and implant-related causes have been described in the literature as potential risk factors for dislocation. The most important patient factors include older age (>70 year), obesity, medical comorbidities, female gender, musculoligamentous laxity, and weakness of the abductors. Cognitive impairment that prevents adequate patient education also plays an important role [1, 3]. In the past decade, several studies concerning the spinopelvic balance have been published identifying the role of spinopelvic stiffness as a unique cause of late dislocation in THA. Spinopelvic stiffness is associated with increased age and increased femoral motion, which may lead to impingement and dislocation [4]. Earlier studies showed an increased hip motion in patients with late dislocations. Heckmann et al. found that a 0.9° increase in femoral occurred motion for every 1.0° loss of pelvic motion, showing the direct influence of spinopelvic stiffness on hip mobility and possible dislocation risk [5]. On the other hand, several surgical and implant-related factors have been described. One of the most debated topics in the current literature is the influence of the surgical approach on dislocation rates. Literature shows evidence of lower dislocation rates when the direct anterior approach is used, compared to the posterior approach, especially when the posterior capsule and short external rotators are not repaired [3, 6, 7]. Other surgical factors such as optimal cup positioning, large femoral heads, elevated acetabular liners, and dual mobility cups were associated with reduced risk of dislocation in multiple studies [8].
Hospital-related factors such as experienced and high-volume surgeons or pre-operative patient education have also been linked with decreased dislocation risk [1]. Although hip dislocation after THA has been well described in the literature, the entrapment of loose bodies causing dislocation is rare. Some cases have been reported in which debris, such as cement or a trochanteric wire caused the dislocation [9, 10]. In this case report however, we describe a case of broken implant trial particles that caused the total hip dislocation. Given the high volume of THA surgeries around the world, understanding of this possible complication is of utmost importance, and we do believe that this case report holds significant educational value.
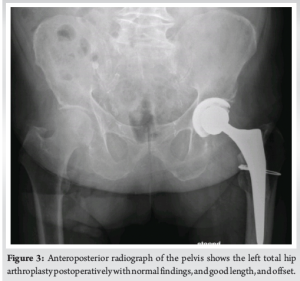
A 67-year-old male patient underwent a hybrid left THA (trabecular metal cup with polyethylene liner, cemented tapered stem, and oxinium head) to treat end stage avascular necrosis (Ficat Stage III). The procedure was conducted through a direct anterior approach. Due to poor bone quality, a cerclage wire was placed at the level of the calcar to prevent periprosthetic fracture during broaching. Perioperative fluoroscopy imaging showed excellent component positions. The procedure was uneventful and the patient was discharged from the hospital after 3 days. 4 weeks after surgery, the patient was referred to the hospital with an anterior hip dislocation following minor rotational trauma. Clinical examination revealed a shortened and externally rotated left leg, and the radiograph confirmed the diagnosis of an anterior hip dislocation. Under general anesthesia, the hip was successfully reduced and subsequently showed an excellent range of motion and stability. The patient subsequently made a full recovery and returned to his usual daily activities. However, 4 weeks after his initial dislocation, the patient sustained a new trauma resulting in a second anterior hip dislocation. Aside from the expected anterior hip dislocation, the radiograph now showed a radiopaque particle between the acetabular cup and the femoral stem. The hip was again successfully reduced under general anesthesia. An additional computed tomography (CT) scan was performed to evaluate the acetabular and femoral component orientation. These were within normal ranges (44° cup inclination, 15° of cup anteversion, and 6° of femoral anteversion). Surprisingly, two radio-opaque particles were visualized on the CT scan. One was projected inside the acetabular cup and the other was visualized directly superior to the femoral stem (Fig. 1). An open exploration and implant inspection were performed through the direct anterior approach. After anterior capsulotomy, one dense plastic particle was immediately visualized and resected. Once the hip was perioperatively dislocated, the second particle became visible, located between the polyethylene liner and the oxinium head. Clear scratch marks were found on the polyethylene liner. The particles were later identified as parts of the implant head trials used, during the initial surgery (Fig. 2). The damaged polyethylene liner and femoral head were replaced without any issues. The patient was ambulating the day after the procedure and was discharged from the hospital after 2 days. No clinical or radiographic complications occurred 3 months and 1 year postoperatively (Fig. 3).
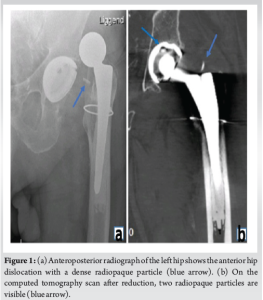

Dislocation following THA is often a painful and dramatic event, and different risk factors have been identified. However, the cause is often multifactorial. Dislocation rates vary in the literature, with some articles suggesting rates up to 10% after primary THA and up to 28% in revisions [1, 3]. In most cases, successful initial management consists of closed reduction under sedation or general anesthesia. Patients with recurrent dislocations often require revision surgery. Only a few cases have been previously reported where the presence of loose intra-articular bodies was responsible for the THA dislocation. Nordt et al. described a case in which loose bodies after THA dislocation were removed arthroscopically [9]. Vakili et al., reported three cases in which bone cement or a trochanteric wire was assumed to cause a THA dislocation [10]. However, loose intra-articular bodies originating from a trial femoral head (TFH) have never been identified as a potential risk factor for dislocation in the literature. In this case, one would expect an eccentric position of the femoral head on perioperative fluoroscopy, but this was not visible. This two-dimensional image of a three-dimensional reality could, however, hide the eccentric anteroposterior position. The CT-scan performed after the first dislocation visualized the two prominent solid radio-opaque pieces but did not demonstrate an eccentric position of the femoral head in the acetabulum, possibly because these particles were partially lodged into the polyethylene.
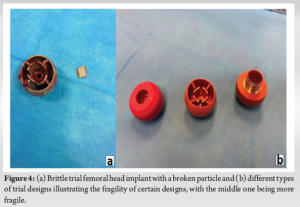
A similar event occurred in another patient a few months after this described case. During a routine THA procedure, a TFH was damaged during perioperative testing. At this time, the surgeon was consistently checking all the used trials and the components could easily be completely removed (Fig. 4). This highlights the importance of a thorough investigation of intraoperative trial components, especially when they are assumed to be made of fragile materials such as plastic, even when no intraoperative abnormalities are present during testing.
We believe that the brittle design of these THA trial components makes them vulnerable to damage during perioperative testing; therefore, care must be taken to check the integrity of these devices during surgery. Surgeons should be aware of this situation and are advised to inspect the integrity of the TFH before wound closure and the sign-out procedure. Even when a dislocation does not occur, loose plastic particles could dramatically damage the polyethylene and jeopardize the durability of the THA. We would like to provide insights for both the surgeon and the manufacturers.
The design of trial implants can be brittle. Therefore, it is vital to inspect surgical instruments for any damaged components before concluding the procedure. Regarding the manufacturers, we recommend producing THF with a design that exhibits a reduced risk of damage during insertion.
References
- 1.Dargel J, Oppermann J, Bruggemann GP, Eysel P. Dislocation following total hip replacement. Dtsch Arztebl Int 2014;111:884-90. [Google Scholar]
- 2.Brooks PJ. Dislocation following total hip replacement: Causes and cures. Bone Joint J 2013;95-B 11 Suppl A:67-9. [Google Scholar]
- 3.Kunutsor SK, Barrett MC, Beswick AD, Judge A, Blom AW, Wylde V, et al. Risk factors for dislocation after primary total hip replacement: Meta-analysis of 125 studies involving approximately five million hip replacements. Lancet Rheumatol 2019;1:e111-21. [Google Scholar]
- 4.Pagan CA, Karasavvidis T, Vigdorchik JM, DeCook CA. Spinopelvic motion: A simplified approach to a complex subject. Hip Pelvis 2024;36:77-86. [Google Scholar]
- 5.Heckmann N, McKnight B, Stefl M, Trasolini NA, Ike H, Dorr LD. Late dislocation following total hip arthroplasty: Spinopelvic imbalance as a causative factor. J Bone Joint Surg Am 2018;100:1845-53. [Google Scholar]
- 6.Hailer NP, Weiss RJ, Stark A, Kärrholm J. The risk of revision due to dislocation after total hip arthroplasty depends on surgical approach, femoral head size, sex, and primary diagnosis. An analysis of 78,098 operations in the Swedish Hip Arthroplasty Register. Acta Orthop 2012;83:442-8. [Google Scholar]
- 7.Tsukada S, Wakui M. Lower dislocation rate following total hip arthroplasty via direct anterior approach than via posterior approach: Five-year-average follow-up results. Open Orthop J 2015;9:157-62. [Google Scholar]
- 8.Scheerlinck T. Cup positioning in total hip arthroplasty. Acta Orthop Belg 2014;80:336-47. [Google Scholar]
- 9.Nordt W, Giangarra CE, Levy IM, Habermann ET. Arthroscopic removal of entrapped debris following dislocation of a total hip arthroplasty. Arthroscopy 1987;3:196-8. [Google Scholar]
- 10.Vakili F, Salvati EA, Warren RF. Entrapped foreign body within the acetabular cup in total hip replacement. Clin Orthop Relat Res 1980;150:159-62. [Google Scholar]