The study concluded that while TXA is effective in minimizing blood loss in TKA with kinematic alignment, additional doses beyond a single dose did not significantly improve outcomes.
Dr. Udit Agrawal, Department of Paediatric Orthopaedics, King George Medical University, Lucknow, Uttar Pradesh, India. E-mail: uditagrawal28@gmail.com
Introduction: Total knee arthroplasty (TKA) is a highly effective treatment for patients with severe knee osteoarthritis, but it often results in significant blood loss, which can lead to complications. Tranexamic acid (TXA) is widely used to reduce blood loss during surgeries, including TKA. However, its effectiveness in the context of kinematic alignment without a tourniquet has not been well-studied, particularly within the Indian population.
Objective: This study aimed to evaluate the impact of multiple intravenous doses of TXA on blood loss, transfusion requirements, and hospital stays in patients undergoing TKA with kinematic alignment without a tourniquet.
Materials and Methods: A randomized controlled trial was conducted with 60 patients aged 50–75 years undergoing unilateral primary TKA. Participants were randomly assigned to three groups: Group A (single dose of TXA and two doses of saline), Group B (single dose of TXA and two doses of TXA), and Group C (three doses of TXA). Blood loss, hemoglobin (Hb) levels, packed cell volume (PCV), and length of hospital stay were measured preoperatively and on post-operative days 1 and 3.
Results: The results showed a significant reduction in blood loss across all groups, with Group C (multiple doses of TXA) showing the greatest reduction in Hb and PCV levels, though no significant differences were found between groups. The total blood loss and maximum Hb drop were similar between groups. No patients required blood transfusions, and there were no major complications such as deep vein thrombosis or pulmonary embolism. Length of hospital stay was not significantly different between groups.
Conclusion: Multiple intravenous doses of TXA in patients undergoing TKA with kinematic alignment and without a tourniquet reduced blood loss, but the reduction was less than reported in studies involving conventional TKA with tourniquet use. Additional doses of TXA did not significantly affect blood loss, transfusion requirements, or hospital stay. Further research with larger sample sizes is needed to confirm these findings and explore optimal strategies for blood management in TKA.
Keywords: Tranexamic acid, total knee arthroplasty, kinematic alignment, blood loss, hospital stay.
In patients with severe osteoarthritis of the knee, total knee replacement (TKR) has been demonstrated to be an exceptionally effective intervention for pain management, functional enhancement, and overall quality of life improvement. The current research objectives regarding TKR focus on minimizing post-operative complications while optimizing patient function and satisfaction. One significant area of concern is the blood loss associated with the procedure. Patients undergoing TKR may experience blood loss ranging from 800 mL to 1,724 mL, which can account for nearly one-third of their total blood volume [1]. This considerable loss is primarily attributed to extensive soft tissue release and bone cuts during surgery. The risks associated with such blood loss are both local and systemic [2]. Tranexamic acid (TXA) has maintained significant interest in the fields of medicine and surgery since its discovery over six decades ago [3]. A considerable number of studies have been conducted in the past 20 years, investigating various administration routes, including intravenous, intra-articular, topical, periarticular, and oral methods [4-6]. These studies have also examined a range of dosages – high doses exceeding 20 mg/kg and low doses below 20 mg/kg – as well as different dosing regimens, including single and multiple doses, along with combinations of these approaches [7, 8]. It is hypothesized that the fibrinolytic system is activated when a tourniquet is inflated [9]. Furthermore, there is emerging evidence that the localized enhancement of fibrinolysis induced by the tourniquet may influence hemostasis for a duration that extends well beyond the surgical procedure itself [10]. Consequently, it is posited that the continuation of antifibrinolytic therapy during the recovery phase may provide additional benefits. This notion is supported by similar findings in studies conducted by Tanaka et al. [9] and Jansen et al. [11]. Recently, the kinematic alignment technique for total knee arthroplasty (TKA), utilizing patient-specific instrumentation, has emerged. This approach minimizes soft tissue release, reduces blood loss, promotes early rehabilitation, and shortens operative time, as indicated by various studies [12, 13]. However, there exists a considerable dearth of literature addressing this issue, particularly within the Indian population, as no known studies have compared the impact of TXA on controlling blood loss during TKR procedures performed using kinematic alignment technique without the use of a tourniquet. This study is initiated to address this gap in the existing literature and to enhance our understanding of the effects of TXA in this specific context.
This study was a single-center, double-blind, randomized controlled trial conducted at the Department of Orthopaedic Surgery, Vivekanand Polyclinic and Institute of Medical Sciences in Lucknow, Uttar Pradesh, India, from September 2017 to May 2018. The institutional review board approved the study protocol, and all participants provided written informed consent before enrolment. The inclusion criteria for the study were male and female patients aged 50–75 years who underwent primary, unilateral TKA due to osteoarthritis of the knee (primary or secondary to RA), with an ASA status of 1–3 and who provided written informed consent to participate. The exclusion criteria for this study were as follows: Patients with anemia, defined as hemoglobin (Hb) levels ≤10 g/dL for females and ≤11 g/dL for males, were excluded. Individuals with a history of thromboembolic events, such as deep vein thrombosis (DVT) or pulmonary embolism (PE), were not eligible for inclusion. Those with a history of myocardial infarction within the past 6 months were also excluded, as were patients with known clotting disorders or hypersensitivity to TXA or related medications. In addition, patients with pathological or congenital malformations of the spine or lower limbs, as well as those with hepatic or renal impairments, and patients with flexion deformity ≥30, varus and/or valgus deformity ≥30 were also excluded from the study. The sample size for this study was calculated based on anticipated blood loss, considering a minimum mean difference of 1.0% (the effect size) between groups. A 5% margin of error (Type I error: α = 0.05) and 80% power (Type II error: 1–β = 0.80) were factored into the calculations, resulting in a minimum sample size requirement of 48 participants overall or 16 participants in each group. Eligible patients were randomized into three groups using a computer-generated method, with allocation hidden in sealed envelopes opened immediately before surgery. Patients received TXA as follows:
- Group A: 20 mg/kg of intravenous (IV) TXA 5–10 min before the skin incision, followed by 10 mg/kg of normal saline at 3- and 6-h post-administration.
- Group B: 20 mg/kg of IV TXA 5–10 min before the incision, followed by 10 mg/kg of TXA at 3 h and another 10 mg/kg of normal saline at 6 h.
- Group C: 20 mg/kg of IV TXA 5–10 min before the skin incision, with 10 mg/kg of TXA given at both 3 and 6 h.
All patients received 2 g of topical TXA during surgery.
Before the procedure, each patient underwent a thorough pre-anesthetic evaluation, including clinical assessments and necessary investigations tailored to their individual health status. TKAs were performed under either general or spinal anesthesia, as deemed appropriate by the anesthesia team, with all surgeries conducted by a single senior surgeon in the same operating room. Prophylactic antibiotics (2 g of cefazolin) were administered 30 min before incision, and a tourniquet was not employed. The surgical procedures included a midline incision, medial parapatellar approach, and kinematic balancing techniques. Electrocautery and hemostasis were routinely performed, with minimal soft tissue releases while preserving collateral ligaments and the posterior cruciate ligament. Intramedullary guides were used for femoral preparation, and extramedullary guides were used for tibial preparation, with an autologous bone plug filling the femoral medullary canal before implant cementation. Two grams of TXA were injected into the intraarticular space, and compression bandaging was applied after closure. The criteria for blood transfusion were set at a Hb level of <7 g/dL with symptomatic anemia. All subjects followed a standardized post-operative recovery and rehabilitation program. Perioperative hematocrit (Hct) and Hb levels, together with the coagulation index and renal function, were assessed preoperatively and on post-operative days 1 and 3. The Nadler formula [14] was utilized to estimate patient blood volume (PBV), while the Gross formula [15] was employed to calculate blood loss based on the PBV and the reduction in Hct levels. Intraoperative blood loss (IBL) was estimated by evaluating the liquid volume in the negative pressure drainage bottle, adding the volume of liquid absorbed by gauze, and subtracting the amount of saline administered. It is important to note that a saturated gauze typically contains approximately 20 mL of liquid. The formula for calculating PBV is expressed as follows: PBV = K1 × height^3 (m³) + K2 × weight (kg) + K3, for male patients, K1 = 0.3669, K2 = 0.03219, and K3 = 0.6041 and for female patients, K1 = 0.3561, K2 = 0.03308, and K3 = 0.1833. The total red blood cell loss (TBL) is calculated using the following formula: TBL = PBV × (Hct_pre – Hct_post)/Hct_avg, where Hct_pre represents the initial preoperative Hct level, Hct_post denotes the Hct level on the third postoperative day, and Hct_avg signifies the average of Hct_pre and Hct_post. Patients were carefully monitored for adverse events, including DVT, PE, wound complications, infections, and acute renal failure. The transfusion rate and incidence of adverse events were evaluated postoperatively during the inpatient hospital stay. Data were summarized as mean ± standard error. Group comparisons were conducted using one-way analysis of variance (ANOVA) or repeated measures two-factor ANOVA. The significance of mean differences both between and within groups was assessed using the Tukey honestly significant difference post hoc test, following verification of normality through the Shapiro–Wilk test and assessment of homogeneity of variance using Levene’s test. Categorical data were analyzed through the Chi-square (χ2) test. A two-tailed P < 0.05 was deemed statistically significant. All statistical analyses were performed utilizing SPSS software version 25.0.
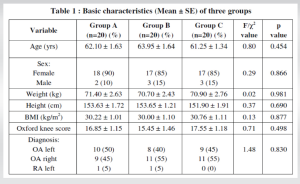
A total of 60 patients were included in this study and divided into the study groups, 20 patients in each group. The analysis of the basic demographic characteristics of the groups, as well as the preoperative health status of the patients, indicates their uniformity and excludes the influence of these parameters on further research (Table 1).
Outcome measures
Hb
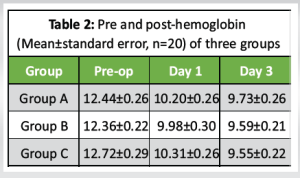
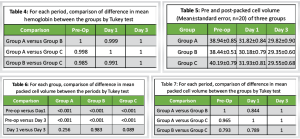
The Hb levels among the three groups are presented in Table 2. An analysis of the data indicates a progressive decrease in mean Hb levels over time. Notably, Group C exhibited the most substantial decline, while Group A demonstrated the least reduction, with Group B falling in between. According to the Tukey test, there was a statistically significant decrease in Hb (P < 0.001) at both day 1 and day 3 when compared to pre-operative (pre-op) levels across all three groups, as detailed in Table 3. However, no significant differences (P > 0.05) were found in Hb levels between day 1 and day 3 within any of the groups. In addition, the comparison of mean Hb levels between the groups revealed no significant differences (P > 0.05) at any of the time points assessed, as shown in Table 4. At the final evaluation, the mean decrease in Hb from pre-op to day 3 was the highest in Group C at 24.9%, followed by Group B at 22.4%, and the least decrease was observed in Group A at 21.8%.
Packed cell volume (PCV)
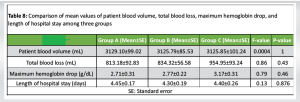
The PCV of the three groups is presented in Table 5. Consistent with the findings related to Hb, the mean PCV exhibited a decline over time, with the most pronounced decrease occurring in Group C, followed by Group B, and the least reduction noted in Group A. A comparison of the mean PCV within each group, as determined by the Tukey test, revealed a statistically significant decrease (P < 0.001) at both day 1 and day 3 when compared to the pre-operative values across all three groups (Table 6). However, the analysis indicated no significant differences (P > 0.05) in PCV between day 1 and day 3. Furthermore, the comparison of mean PCV between the groups demonstrated no significant differences (P > 0.05) at any of the assessed time points, as shown in Table 7. At the final evaluation, the reduction in mean PCV from pre-operative to day 3 was greatest in Group C, at 26.5%, followed by Group B at 23.6%, with Group A exhibiting the least change at 23.4%. The additional outcome measures that were recorded included PBV, total blood loss, maximum Hb drop, and length of hospital stay, which have been summarized in Table 8. A comparison of the means across all three groups, conducted through ANOVA, indicated that the values were consistent among the groups and did not differ significantly. None of the patients included in the study required blood transfusions, and there were no reported incidents of DVT or PE among the participants. All patient groups received prophylactic measures for DVT, which consisted of sequential compression devices, and chemoprophylaxis was employed to mitigate the risk of venous thromboembolism. Furthermore, during the 30-day follow-up period, no additional adverse events were observed, including cardiac infarction, stroke, acute renal failure, or wound-related complications.
The rate of blood transfusions after conventional TKR is about 24%, primarily due to significant intraoperative and postoperative bleeding [16]. Studies indicate that transfusions increase the risk of infections, thrombosis, adverse immunologic reactions, and higher procedure costs [17, 18]. To reduce the need for transfusions, strategies such as pneumatic above-the-knee tourniquets, post-operative blood salvage, hypotensive anesthesia, and topical agents like TXA are employed [19, 20]. However, the use of tourniquets is controversial; while they can enhance visibility and manage blood loss, they also carry risks such as DVT, thigh discomfort, vascular damage, and nerve issues [21]. According to Guler et al. [22], tourniquet use may decrease muscle volume and delay knee function recovery. Conversely, Ejaz et al. [23] have demonstrated that performing TKA without a tourniquet yields better functional and clinical outcomes, such as improved range of motion and a faster recovery rate. TXA plays a role in reducing blood loss by inhibiting the proteolytic activity of plasmin, thereby stabilizing clot formation. Both systemic and topical administrations of TXA have been shown to minimize blood loss during TKA, as indicated in a review by Kim et al. [24]. Fibrinolysis around the surgical site peaks within 6 h and is maintained for about 18 h. The half-life of TXA in plasma is 2–3 h, and its antifibrinolytic effects last for approximately 8 h. After intravenous administration, about 90% of the drug is recoverable from urine within 24 h [25, 26]. To maintain an adequate concentration of TXA in the plasma, we repeated intravenous administration of the drug twice postoperatively. Based on both in vivo and in vitro data, the effective therapeutic plasma concentration of TXA needed to inhibit fibrinolysis has been determined to be between 5–10 mg/L and 10–15 mg/L [27, 28]. Our study indicated that the administration of TXA resulted in a reduction of blood loss by 26%–30%. This finding is lower than the reductions reported in previous studies, which noted reductions of 45%–52% in routine TKRs [11, 29]. The observed difference may be attributable to the limited soft tissue release technique or the application of kinematic alignment. The primary function of TXA is to promote hemostasis; however, the restricted soft tissue release, executed through the kinematic balancing technique, limits the areas of effectiveness for TXA, as it spares collateral ligaments, posterior soft tissues, and retinacular ligaments. Moreover, the absence of a tourniquet during the procedure may have contributed to increased fibrinolytic activity. Hemostasis was achieved intraoperatively, from the skin incision through to minimal soft tissue release. The raw surfaces of bone, which represent potential sites of blood loss, were effectively plugged with cement particles, thereby minimizing bleeding. In addition, the femoral canal was routinely filled with bone. The watertight closure of the compartment created a tamponade effect, and the topical administration of TXA in all patients further reduced blood loss. There is a significant amount of literature indicating that the use of a tourniquet, along with multiple doses of TXA, can reduce blood loss and shorten hospital stays for patients undergoing TKA [28,30,31]. However, there is limited data regarding similar studies conducted without the use of a tourniquet. To the best of our knowledge, this is the first randomized controlled study involving the Indian population that examines the efficacy of multiple doses of TXA in patients undergoing TKR surgery using the kinematic alignment technique without employing a tourniquet. Our study concludes that additional intravenous bolus doses of TXA do not lead to a significant reduction in blood loss or a decrease in the length of hospital stay for patients, which contrasts with the findings of the previously available limited research data [32, 33, 34]. This study presents a few limitations that merit consideration. First, it was a single-center investigation with a limited patient sample size. Second, the study was underpowered to make definitive conclusions regarding safety, as thromboembolic complications were monitored solely through clinical parameters, potentially allowing for the omission of some instances. Third, the gravimetric method utilized to estimate blood loss is inherently subject to imprecision; nevertheless, no entirely objective and clinically practical method for measuring IBL is currently available. Fourth, the shorter follow-up period excludes evaluating long-term outcomes such as functional recovery, joint mobility, and patient-reported outcomes. Ethical constraints also precluded using a placebo group, which would have helped assess the actual effectiveness of TXA. Finally, the study’s focus on a specific Indian population and the use of kinematic alignment may limit the broader applicability of the findings to different surgical practices or patient populations.
This randomized controlled study investigated the efficacy of administering multiple doses of intravenous TXA to patients undergoing TKA utilizing the kinematic alignment technique without the application of a tourniquet. The findings revealed that TXA effectively reduced blood loss by approximately 26%–30%. This reduction is notably less than those reported in earlier studies involving conventional TKA procedures with tourniquet usage. Furthermore, the administration of additional doses of TXA did not yield a statistically significant decrease in blood loss or Hb reduction nor did it contribute to a shortened length of hospital stay. This research underscores the notion that, while TXA is beneficial in reducing blood loss, its advantages may be constrained within the context of the kinematic alignment technique and the absence of a tourniquet. Therefore, additional research involving larger sample sizes and multi-center studies is warranted to validate these findings and to investigate further strategies for enhancing blood management in TKA procedures.
This randomized controlled study investigated the effects of multiple intravenous doses of TXA on blood loss, transfusion needs, and hospital stay in patients undergoing TKA with kinematic alignment, without tourniquet use. Sixty patients were divided into three groups receiving varying TXA doses. Results showed a reduction in blood loss across all groups, with the highest reduction in Group C (three doses of TXA). However, no significant differences in blood loss, transfusion requirements, or hospital stays were found between the groups. The study concluded that additional TXA doses had minimal impact on these outcomes.
References
- 1.Sehat KR, Evans R, Newman JH. How much blood is really lost in total knee arthroplasty? Correct blood loss management should take hidden loss into account. Knee 2000;7:151-5. [Google Scholar]
- 2.Lotke PA, Faralli VJ, Orenstein EM, Ecker ML. Blood loss after total knee replacement. Effects of tourniquet release and continuous passive motion. J Bone Joint Surg Am 1991;73-A:1037-40. [Google Scholar]
- 3.Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: A meta-analysis. J Bone Joint Surg Am 2012;94:1153-9. [Google Scholar]
- 4.Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: A systematic review and meta-analysis. J Bone Joint Surg Br 2011;93:1577-85. [Google Scholar]
- 5.Wong J, Abrishami A, El Beheiry H, Mahomed NN, Roderick Davey J, Gandhi R, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: A randomized, controlled trial. J Bone Joint Surg Am 2010;92:2503-13. [Google Scholar]
- 6.Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Pérez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: A double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am 2014;96:1937-44. [Google Scholar]
- 7.Maniar RN, Kumar G, Singhi T, Nayak RM, Maniar PR. Most effective regimen of tranexamic acid in knee arthroplasty: A prospective randomized controlled study in 240 patients. Clin Orthop Relat Res 2012;470:2605-12. [Google Scholar]
- 8.Lu F, Sun X, Wang W, Zhang Q, Guo W. What is the ideal route of administration of tranexamic acid in total knee arthroplasty? A meta-analysis based on randomized controlled trials. Ann Palliat Med 2021;10:1880-94. [Google Scholar]
- 9.Tanaka N, Sakahashi H, Sato E, Hirose K, Ishima T, Ishii S. Timing of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. J Bone Joint Surg Br 2001;83:702-5. [Google Scholar]
- 10.Fu DJ, Chen C, Guo L, Yang L. Use of intravenous tranexamic acid in total knee arthroplasty: A meta-analysis of randomized controlled trials. Chin J Traumatol 2013;16:67-76. [Google Scholar]
- 11.Jansen AJ, Andreica S, Claeys M, D’Haese J, Camu F, Jochmans K. Use of tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. Br J Anaesth 1999;83:596-601. [Google Scholar]
- 12.Thienpont E, Schwab PE, Fennema P. Efficacy of patient-specific instruments in total knee arthroplasty: A systematic review and meta-analysis. J Bone Joint Surg Am 2017;99:521-30. [Google Scholar]
- 13.Roussot M, Vles G, Oussedik S. Clinical outcomes of kinematic alignment versus mechanical alignment in total knee arthroplasty: A systematic review. EFORT Open Rev 2020;5:486-97. [Google Scholar]
- 14.Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery 1962;51:224-32. [Google Scholar]
- 15.Gross JB. Estimating allowable blood loss: Corrected for dilution. Anesthesiology 1983;58:277-80. [Google Scholar]
- 16.Keating EM, Meding JB, Faris PM, Ritter MA. Predictors of transfusion risk in elective knee surgery. Clin Orthop Relat Res 1998;357:50-9. [Google Scholar]
- 17.Bong MR, Patel V, Chang E, Issack PS, Hebert R, Di Cesare PE. Risks associated with blood transfusion after total knee arthroplasty. J Arthroplasty 2004;19:281-7. [Google Scholar]
- 18.Li X, Xie H, Liu S, Wang J, Shi Z, Yao Q, et al. Analysis of the incidence and risk factors of blood transfusion in total knee revision: A retrospective nationwide inpatient sample database study. BMC Musculoskelet Disord 2024;25:225. [Google Scholar]
- 19.Muñoz M, Ariza D, Campos A, Martín-Montañez E, Pavía J. The cost of post-operative shed blood salvage after total knee arthroplasty: An analysis of 1,093 consecutive procedures. Blood Transfus 2013;11:260-71. [Google Scholar]
- 20.Lin S, McKenna SJ, Yao CF, Chen YR, Chen C. Effects of hypotensive anesthesia on reducing intraoperative blood loss, duration of operation, and quality of surgical field during orthognathic surgery: A systematic review and meta-analysis of randomized controlled trials. J Oral Maxillofac Surg 2017;75:73-86. [Google Scholar]
- 21.Ahmed I, Chawla A, Underwood M, Price AJ, Metcalfe A, Hutchinson C, et al. Tourniquet use for knee replacement surgery. Cochrane Database Syst Rev 2020 Dec 8;12(12):CD012874. [Google Scholar]
- 22.Guler O, Mahirogullari M, Isyar M, Piskin A, Yalcin S, Mutlu S, et al. Comparison of quadriceps muscle volume after unilateral total knee arthroplasty with and without tourniquet use. Knee Surg Sports Traumatol Arthrosc 2016;24:2595-605. [Google Scholar]
- 23.Ejaz A, Laursen AC, Kappel A, Laursen MB, Jakobsen T, Rasmussen S, et al. Faster recovery without the use of a tourniquet in total knee arthroplasty. Acta Orthop 2014;85:422-6. [Google Scholar]
- 24.Kim TK, Chang CB, Koh IJ. Practical issues for the use of tranexamic acid in total knee arthroplasty: A systematic review. Knee Surg Sports Traumatol Arthrosc 2014;22:1849-58. [Google Scholar]
- 25.Kim C, Park SS, Davey JR. Tranexamic acid for the prevention and management of orthopedic surgical hemorrhage: Current evidence. J Blood Med 2015;6:239-44. [Google Scholar]
- 26.Blanié A, Bellamy L, Rhayem Y, Flaujac C, Samama CM, Fontenay M, et al. Duration of postoperative fibrinolysis after total hip or knee replacement: A laboratory follow-up study. Thromb Res 2013;131:e6-11. [Google Scholar]
- 27.Li MM, Kwok JY, Chung KY, Cheung KW, Chiu KH, Chau WW, et al. Prospective randomized trial comparing efficacy and safety of intravenous and intra-articular tranexamic acid in total knee arthroplasty. Knee Surg Relat Res 2020;32:62. [Google Scholar]
- 28.Kang BX, Xu H, Gao CX, Zhong S, Zhang J, Xie J, et al. Multiple intravenous tranexamic acid doses in total knee arthroplasty in patients with rheumatoid arthritis: A randomized controlled study. BMC Musculoskelet Disord 2021;22:425. [Google Scholar]
- 29.Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: A prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg Br 1996;78:434-40. [Google Scholar]
- 30.Konarski W, Poboży T, Hordowicz M. Tranexamic acid in total knee replacement and total hip replacement - a single-center retrospective, observational study. Orthop Rev (Pavia) 2022;14:33875. [Google Scholar]
- 31.Jovanovic G, Lukic-Sarkanovic M, Lazetic F, Tubic T, Lendak D, Uvelin A. The effect of intravenous tranexamic acid on perioperative blood loss, transfusion requirements, verticalization, and ambulation in total knee arthroplasty: A randomized double-blind study. Medicina (Kaunas) 2024;60:1183. [Google Scholar]
- 32.Tzatzairis TK, Drosos GI, Kotsios SE, Ververidis AN, Vogiatzaki TD, Kazakos KI. Intravenous vs topical tranexamic acid in total knee arthroplasty without tourniquet application: A randomized controlled study. J Arthroplasty 2016;31:2465-70. [Google Scholar]
- 33.Xie J, Ma J, Yao H, Yue C, Pei F. Multiple boluses of intravenous tranexamic acid to reduce hidden blood loss after primary total knee arthroplasty without tourniquet: A randomized clinical trial. J Arthroplasty 2016;31:2458-64. [Google Scholar]
- 34.Bidolegui F, Arce G, Lugones A, Pereira S, Vindver G. Tranexamic acid reduces blood loss and transfusion in patients undergoing total knee arthroplasty without tourniquet: A prospective randomized controlled trial. Open Orthop J 2014;8:250-4. [Google Scholar]