- Cutibacterium acnes can cause clavicular osteomyelitis even in the absence of a history of prior surgery - Adequate debridement is essential in managing osteomyelitis - Dead space filling with antibiotic-impregnated calcium sulfate beads has a potential therapeutic effect
Dr. Ramy Samargandi, Department Orthopedic Surgery, College of Medicine, University of Jeddah, Jeddah, Saudi Arabia. E-mail: rsamargandi@uj.edu.sa
Introduction: Osteomyelitis due to Cutibacterium acnes in the clavicle without a history of previous surgery is extremely rare and has been reported in one previous study. In this report, we delve into a case of clavicular osteomyelitis caused by C. acnes without the presence of hardware materials.
Case Report: We report here a case of a 32-year-old female presented with spontaneous clavicular osteomyelitis due to C. acnes that failed with medical management. The Patient was successfully treated by surgical debridement and calcium sulfate filling that impregnated with local antibiotics.
Conclusion: This case demonstrates that clavicular osteomyelitis can be caused by C. acnes even without the presence of a device. Therefore, cultures from potential bone infections that yield C. acnes should not be dismissed as contaminants. Combining clinical and laboratory criteria with emerging microbiologic tests may enhance the predictive value of C. acnes diagnostic testing in the future.
Keywords: Bone infection, clavicular infection, Cutibacterium acnes, debridement, calcium sulfate, osteomyelitis.
Cutibacterium acnes is a slow-growing, Gram-positive bacterium commonly found in the normal skin flora of the shoulder and chest, as highlighted in orthopedic studies [1,2]. Its high prevalence in the shoulder area makes it a significant pathogen for post-operative infections, particularly in prosthetic shoulder implants and other orthopedic device-related infections of the upper limb [3,4]. The clinical progression is slow and does not exhibit typical infection symptoms, initially presenting with shoulder pain and stiffness, and later progressing to local swelling or redness [2-4]. Several reports have been published regarding clavicular infection due to C. acnes after previous surgery and hardware implantation or due to direct colonization in asymptomatic patients [5-7]. However, osteomyelitis of the clavicle due to C. acnes in the absence of a history of surgery or hardware materials is extremely rare. To our knowledge, only one previous report has described two cases of osteomyelitis due to C. acnes not associated with implants [8]. In this report, we present a case of a female patient with spontaneous clavicular osteomyelitis secondary to C. acnes infection without a history of previous surgery in the upper limb.
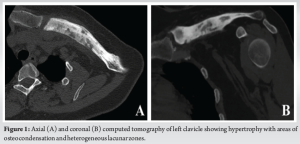
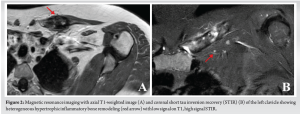
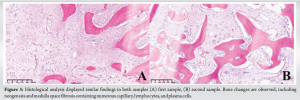
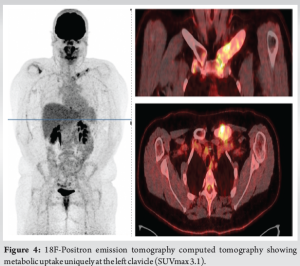
In 2018, a 32-year-old female presented due to left clavicle pain. Her history included diagnosed psoriasis in youth, no other medical or surgical history, three vaginal deliveries (G3 P3), and no allergies. The patient had no clear risk factors for developing osteomyelitis and no history of trauma. The patient presented with symptoms that were inflammatory in nature with initial swelling and night sweats without fever. Symptoms had been evolving for approximately 3 years, postpartum, affecting only the left clavicle. A computed tomography (CT) scan revealed an abnormality in the bone structure, showing hypertrophy with areas of osteo-condensation and heterogeneous lacunar zones, associated with muscle inflammation in contact (Fig. 1). The magnetic resonance imaging revealed osteitis in the medial and middle thirds of the left clavicle, with heterogeneous hypertrophic inflammatory bone remodeling (low T1 signal, high short tau inversion recovery signal), and moderate peri-lesional soft tissue infiltration without organized collection (Fig. 2). The patient underwent a biopsy for pathological examination and bacterial culture, which revealed non-specific osteitis at varying stages, from acute to chronic subacute (Fig. 3), with C. acnes detected in 4 out of 5 bacterial cultures. She was then treated with clindamycin 600 mg 3 times/day for a duration of 6 weeks. The patient showed dramatic improvement, with the pain disappearing, but has been lost to follow-up since 2018. 5 years later, in 2023, she came with a recurrence of pain in the left clavicle. A biopsy was then conducted which revealed bone tissue site of non-specific chronic osteitis lesions, absence of progressive inflammatory process, absence of element suspicious for malignancy, and Cutibacterium was found again in five out of five culture samples. The patient also underwent a 18 F-fluorodeoxyglucose positron emission tomography (PET) scan to rule out other differential diagnosis such as synovitis, acne, pustulosis, hyperostosis, and osteitis (SAPHO) syndrome, chronic recurrent multifocal osteomyelitis (CRMO). PET-CT revealed metabolic hyperactivity uptake only at the clavicle (SUVmax 3.1), confirming the unique localization and excluding other differential diagnosis (Fig. 4).
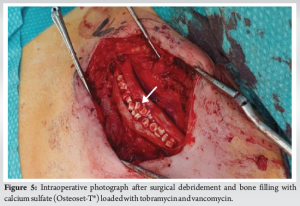
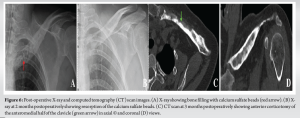
A new surgical bone biopsy, including five bacteriological samples, mycobacterial cultures, and a histopathological sample, was performed at zones of hypermetabolism identified by PET scan. The histopathological results were consistent with previous findings, while mycobacterial cultures were negative. However, all C. acnes cultures were positive 5/5. Due to the failure of non-surgical management, the patient underwent extensive surgical debridement. A sclerotic appearance without the appearance of bleeding bone was found. A horizontal anterior corticotomy for bone decompression was performed, followed by mechanical debridement with a high-speed burr and copious irrigation. The dead space was then filled with calcium sulfate Osteoset‐T® (Wright Medical Technology), loaded with tobramycin and 1 g of vancomycin, and a drain was placed (Fig. 5). Postoperatively, the patient was prescribed empirical antibiotics, including piperacillin-tazobactam 4 g intravenous (IV) 3 times/day and linezolid 600 mg IV twice daily. Assessment of the culture revealed for the third time that C. acnes samples were all positive (5/5). Afterward, the patient was treated with amoxicillin 2 g 3 times/day for 3 months, and symptoms had completely resolved by the 5-month follow-up. The calcium sulfate beads were fully resorbed 2 months postoperatively, indicating successful integration and healing (Fig. 6).
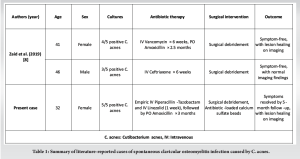
Diagnosing bone infections caused by low-virulence organisms, such as C. acnes, is challenging due to the lack of typical clinical signs of disease and the difficulties in confirming the diagnosis due to the possible contaminations [9]. The incidence of bone infection without the presence of an implant is believed to be much lower [6]. What is unique in this case is that the clavicular infection caused by C. acnes occurred without a history of prior surgery, underscoring the importance of considering this organism as a potential pathogen in bone infections, even in the absence of a metallic device. This finding is consistent with a previous study that reported C. acnes clavicular infection in the absence of an implant [8]. Positive cultures are sometimes dismissed as “contaminants,” “false-positives,” or non-pathogenic Cutibacterium “growth in deep-tissue layers” [10]. In addition, certain infections can easily mimic malignancies, hence it is logical to consider malignancies as a differential diagnosis in such case. Tissue sampling and histopathological examination are essential to distinguish such lesions and to choose the right treatment. However, in our study, this organism was cultured multiple times on separate occasions. A CT scan revealed bone structure abnormalities, showing hypertrophy with areas of osteocondensation and heterogeneous lacunar zones, along with muscle inflammation in contact. PET-CT helps in evaluating the absence of other localization to exclude SAPHO and CRMO. Furthermore, it aids in identifying zones of hypermetabolism at the lesion which helps the surgeon to meticulously debride all active zones [11]. Conditions to consider in the differential diagnosis for a patient with clavicular pain and radiographic changes include malignancy, SAPHO, condensing osteitis, Friedrich’s disease, sternoclavicular hyperostosis, and CRMO [12,13]. Unlike C. acnes osteomyelitis, condensing osteitis is self-limiting and should resolve spontaneously within months. Friedrich’s disease, or osteonecrosis of the medial clavicle, is another rare condition that can present with localized pain. In this study, the patient required extensive surgical debridement and antibiotic therapy, which is necessary in clavicular infections. C. acnes is known to be difficult to eradicate, partly due to its biofilm production, which may protect against host immune responses and antimicrobial therapy [14]. Evidence suggests that management should include aggressive irrigation and debridement, removal of any hardware, and extended antibiotic treatment. This is also compatible with clavicular infection with the presence of an implant, where in a previous case study, it was reported that the patient underwent implant removal revision surgery and additional IV antibiotics [5]. Similar to our case, Zaid et al. reported a case of a 41-year-old woman with right clavicular osteomyelitis caused by C. acnes, confirmed by intraoperative cultures. The initial treatment included surgical debridement and 6 weeks of IV ceftriaxone followed by oral amoxicillin. The patient developed a recurrence of the infection, requiring a second debridement and IV vancomycin followed by oral amoxicillin. At the 2-year and 3-month follow-up, she remained asymptomatic. They reported another case of a 46-year-old man with right clavicular osteomyelitis caused by C. acnes. The patient was treated with surgical debridement followed by 6 weeks of IV ceftriaxone. The patient was asymptomatic at 2-year follow-up [8]. Table 1 summarizes literature-reported cases of spontaneous clavicular osteomyelitis infection caused by C. acnes.
In the current study, the patient was successively managed by surgical debridement in conjunction with dead space filling by calcium sulfate (Osteoset‐T®) and antibiotic therapy for 3-month duration. Calcium sulfate is acknowledged for its key features such as high biocompatibility, and osteoconductive properties by fast rate of resorption, stimulates osteogenesis, and reducing the local pH in the target area, which in turn leads to local bone mineralization [15]. It also has an advantage of delivering a high concentration of antibiotics and providing a scaffold for new bone formation, therefore playing a role in spontaneous bone regeneration which can be assessed radiographically [16]. In addition, it was claimed that antibiotics administered to patients infected with C. acnes retain their activity when mixed with calcium sulfate, a finding reported in a previous study by Couture et al. [17]. Previous studies have demonstrated the efficacy of calcium sulfate beads in the treatment of osteomyelitis. Ferguson et al. treated 195 patients with chronic osteomyelitis using antibiotic-loaded calcium sulfate beads, successfully resolving infections in 90.8% of cases [18]. Another study published by Zhou et al. treated 38 cases of chronic tibial osteomyelitis using local debridement combined with antibiotic-loaded calcium sulfate, achieving infection remission without recurrence in 88.4% of cases [19]. Similarly, Ferrando et al. and Badie and Arafa reported successful outcomes using antibiotic-loaded calcium sulfate beads in the treatment of chronic osteomyelitis, with infection eradication achieved in 92.3% and 76.7% of cases, respectively [20,21]. In a randomized controlled trial by McKee et al., Osteoset-T® achieved an 86% infection eradication rate in chronic osteomyelitis and infected non-unions [22]. Similarly, a retrospective review by Humm et al. reported infection eradication in 20 of 21 chronic tibial osteomyelitis cases treated with Osteoset-T® [23].
While most C. acnes infections occur following the placement of an orthopedic implant, the absence of previous surgery does not exclude the possibility of a C. acnes bone infection. Our case demonstrates that clavicular osteomyelitis can be caused by C. acnes even without the presence of a hardware material. Therefore, cultures from potential bone infections that yield C. acnes should not be dismissed as contaminants. Diagnosis comprises fulfilling both clinical and laboratory criteria, strengthened by the emerging microbiologic tests which may improve the diagnostic capability in the future.
Clavicular osteomyelitis due to C. acnes without prior surgery is extremely rare, with only one previous study reported. Most cases involve prosthetic devices. This report presents a case of clavicular osteomyelitis caused by C. acnes without hardware, treated with surgical debridement in conjunction with calcium sulfate filling for its potential therapeutic benefits.
References
- 1.Achermann Y, Goldstein EJ, Coenye T, Shirtliffa ME. Propionibacterium acnes: From commensal to opportunistic biofilm-associated implant pathogen. Clin Microbiol Rev 2014;27:419-40. [Google Scholar | PubMed]
- 2.Dodson CC, Craig EV, Cordasco FA, Dines DM, Dines JS, Dicarlo E, et al. Propionibacterium acnes infection after shoulder arthroplasty: A diagnostic challenge. J Shoulder Elbow Surg 2010;19:303-7. [Google Scholar | PubMed]
- 3.Kajita Y, Iwahori Y, Harada Y, Takahashi R, Deie M. Incidence of Cutibacterium acnes in open shoulder surgery. Nagoya J Med Sci 2021;83:151-7. [Google Scholar | PubMed]
- 4.Jacquot A, Samargandi R, Peduzzi L, Mole D, Berhouet J. Infected shoulder arthroplasty in patients younger than 60 years: Results of a multicenter study. Microorganisms 2023;11:2770. [Google Scholar | PubMed]
- 5.Washburn F, Tran B, Golden T. Occult clavicle osteomyelitis caused by Cutibacterium acnes (C. acnes) after coracoclavicular ligament reconstruction: A case report and review of the literature. Int J Surg Case Rep 2022;94:107114. [Google Scholar | PubMed]
- 6.Von Keudell AG, Nelson SB, Jupiter JB. CASE SERIES ABSTRACT Proprionibacterium acnes infection complicating the operative treatment of clavicle fractures. [Google Scholar | PubMed]
- 7.Both A, Klatte TO, Lübke A, Büttner H, Hartel MJ, Grossterlinden LG, et al. Growth of Cutibacterium acnes is common on osteosynthesis material of the shoulder in patients without signs of infection. Acta Orthop 2018;89:580-4. [Google Scholar | PubMed]
- 8.Zaid M, Chavez MR, Carrasco AE, Zimel MN, Zhang AL, Horvai AE, et al. Cutibacterium (formerly Propionibacterium) acnes clavicular infection. J Bone Jt Infect 2019;4:40-9. [Google Scholar | PubMed]
- 9.Hoch A, Fritz Y, Dimitriou D, Bossard DA, Fucentese SF, Wieser K, et al. Treatment outcomes of patients with Cutibacterium acnes-positive cultures during total joint replacement revision surgery: A minimum 2-year follow-up. Arch Orthop Trauma Surg 2023;143:2951-8. [Google Scholar | PubMed]
- 10.Moroder P, Trampuz A, Scheibel M. Propionibacterium: We found it, now we have to deal with it: Commentary on an article by Jason E. Hsu, MD, et al.: “Single-stage revision is effective for failed shoulder arthroplasty with positive cultures for Propionibacterium” J Bone Joint Surg 2016;98:e112. [Google Scholar | PubMed]
- 11.Elsheikh A, Elazazy M, Elkaramany M. Role of 18F-FDG PET-CT in pre-operative planning of surgical debridement in chronic osteomyelitis. Indian J Orthop 2022;56:2237-44. [Google Scholar | PubMed]
- 12.Rukavina I. SAPHO syndrome: A review. J Child Orthop 2015;9:19-27. [Google Scholar | PubMed]
- 13.Levy M, Goldberg I, Fischel RE, Frisch E, Maor P. Friedrich’s disease. Aseptic necrosis of the sternal end of the clavicle. J Bone Joint Surg Br 1981;63B:539-41. [Google Scholar | PubMed]
- 14.Coenye T, Peeters E, Nelis HJ. Biofilm formation by Propionibacterium acnes is associated with increased resistance to antimicrobial agents and increased production of putative virulence factors. Res Microbiol 2007;158:386-92. [Google Scholar | PubMed]
- 15.Tayshetye RS, Bhola N, Deshpande N, Agrawal A. Efficacy of calcium sulfate dihydrate as a bone graft substitute in odontogenic cystic defects of jaws following enucleation: A clinical study. Natl J Maxillofac Surg 2023;14:125-9. [Google Scholar | PubMed]
- 16.Sheikh Z, Sima C, Glogauer M. Bone replacement materials and techniques used for achieving vertical alveolar bone augmentation. Materials (Basel) 2015;8:2953-93. [Google Scholar | PubMed]
- 17.Couture A, Lavergne V, Sandman E, Leduc JM, Benoit B, Leduc S, et al. Calcium sulphate mixed with antibiotics does not decrease efficacy against Cutibacterium acnes (formerly Propionibacterium acnes), In vitro study. J Orthop 2019;19:138-42. [Google Scholar | PubMed]
- 18.Ferguson JY, Dudareva M, Riley ND, Stubbs D, Atkins BL, McNally MA. The use of a biodegradable antibiotic-loaded calcium sulphate carrier containing tobramycin for the treatment of chronic osteomyelitis: A series of 195 cases. Bone Joint J 2014;96-B:829-36. [Google Scholar | PubMed]
- 19.Zhou CH, Ren Y, Ali A, Meng XQ, Zhang HA, Fang J, et al. Single-stage treatment of chronic localized tibial osteomyelitis with local debridement and antibiotic-loaded calcium sulfate implantation: A retrospective study of 42 patients. J Orthop Surg Res 2020;15:201. [Google Scholar | PubMed]
- 20.Ferrando A, Part J, Baeza J. Treatment of cavitary bone defects in chronic osteomyelitis: Bioactive glass S53P4 vs. Calcium sulphate antibiotic beads. J Bone Jt Infect 2017;2:194-201. [Google Scholar | PubMed]
- 21.Badie AA, Arafa MS. One-stage surgery for adult chronic osteomyelitis: Concomitant use of antibiotic-loaded calcium sulphate and bone marrow aspirate. Int Orthop 2019;43:1061-70. [Google Scholar | PubMed]
- 22.McKee MD, Li-Bland EA, Wild LM, Schemitsch EH. A prospective, randomized clinical trial comparing an antibiotic-impregnated bioabsorbable bone substitute with standard antibiotic-impregnated cement beads in the treatment of chronic osteomyelitis and infected nonunion. J Orthop Trauma 2010;24:483-90. [Google Scholar | PubMed]
- 23.Humm G, Noor S, Bridgeman P, David M, Bose D. Adjuvant treatment of chronic osteomyelitis of the tibia following exogenous trauma using OSTEOSET(®)-T: A review of 21 patients in a regional trauma centre. Strategies Trauma Limb Reconstr 2014;9:157-61. [Google Scholar | PubMed]