This article reviews the existing literature on osteomyelitis of the odontoid process, highlighting trends in patient presentation, diagnostic modalities, treatment, and outcomes.
Mr. Justin Choi, M.Ed., Department of Otolaryngology, SUNY Downstate Health Sciences University, Brooklyn - 11203, New York, United States. E-mail: Justin.choi@downstate.edu
Introduction: Odontoid osteomyelitis (OOM) is a rare clinical entity that requires a high degree of suspicion for diagnosis. A literature review was conducted to make recommendations for early diagnosis and best practices in the management of OOM.
Materials and Methods: Literature review of all available articles, all of which were case studies or case series, published in PubMed and Google Scholar.
Results: There were 47 articles published from 1944 to 2024 with n = 54 with confirmed diagnosis of OOM. The median age was 52 years and 64% were male. Likely precipitating factors were identified in 29 patients (54%). Neck pain was the most common presentation (87%), followed by fever (57%) and neck stiffness (44%). Neurological symptoms developed in 50% of patients. Magnetic resonance imaging (MRI) was frequently used (85%) with 72% of these indicating OOM. Staphylococcus aureus was the most common pathogen (82%). Surgery was performed in 59% of patients.
Conclusion: To avoid delayed or misdiagnosis of this rare entity, a thorough history and physical should be performed to identify patient risk factors, salient complaints, and potential nidi of infection. MRI remains the gold standard in diagnosis. Early pathogen identification with appropriate antibiotics, and incision and drainage when possible, can treat OOM while avoiding surgery. However, surgery is indicated in cord compression secondary to atlantoaxial subluxation, a common and feared complication of OOM.
Keywords: Osteomyelitis, odontoid process, dens, axis, endonasal surgery.
Vertebral osteomyelitis is an infection of the spine and comprises 3–5% of all cases of osteomyelitis [1]. While the thoracic and lumbar regions are most affected, infection of the cervical region is rare, occurring in approximately 10% of vertebral osteomyelitis cases [1]. Infection of the upper cervical spine has even lower incidence of <1% of all cases of vertebral osteomyelitis [2]. The odontoid process, also named the dens or peg, is the anterior bony protrusion of the second cervical vertebra (C2 or the axis) that articulates with the anterior arch of the atlas (C1) to permit the full range of motion of the neck. Odontoid osteomyelitis (OOM) is a rare disease, mostly described in the literature through single case reports. Early diagnosis of this entity is difficult due to its location deep within the neck and non-specific presentation. Left untreated however, OOM can result in neurologic defects due to atlantoaxial subluxation (AAS) which can cause spinal cord compression, quadriplegia, and death. Rates of diagnosis of OOM have increased in recent years due in part to improved imaging capabilities, especially that of magnetic resonance imaging (MRI), and an increased number of patients with risk factors for vertebral osteomyelitis, such as advanced age and immunocompromise. Herein, we perform a systematic review of the literature on osteomyelitis of the odontoid process, including clinical presentation, diagnosis, sequelae, and management.
To characterize similarities in patients presenting with OOM to facilitate early diagnosis. To identify best practices in the management of patients with OOM to minimize complications.
PubMed and Google Scholar were systematically searched using “osteomyelitis odontoid process,” “osteomyelitis dens,” and “osteomyelitis odontoid peg.” Titles and abstracts were included for further review if they presented a case of osteomyelitis of the odontoid process or the cervical spine with involvement of the odontoid process. Articles describing osteomyelitis of the cervical spine elsewhere, including the body of C2 without odontoid process involvement, were excluded, as were cases of osteomyelitis due to tuberculosis. PubMed yielded 43 results, of which 40 contained titles and abstracts within scope. Using the same queries in Google Scholar yielded an additional 16 unique articles with titles and abstracts within scope. All articles contained case studies or case series. One article could not be accessed. The remaining articles were assessed in their entirety. Ultimately, there were 47 articles containing case studies of 54 patients.
Case publications ranged from 1944 to 2024. Cases describing infants and children were included (n = 8). A summary of patient demographics, clinical history, and clinical course are provided in Table 1. The overall median age was 52 years (range: 20 days–90 years). Most patients were male (n = 32, 64%) with four infants having no sex described. Eight patients had head-and-neck comorbidity (otitis media [3,4], tonsil/adenoid abscess or hypertrophy [4,5], chronic sinusitis [6], nasopharyngeal carcinoma [7,8], and dental disease [6,9]). Twelve patients had a history of diabetes mellitus [4,6,9-18] and five hypertension [14,15,18,19]. Intravenous (IV) drug use was reported in four patients [13,15,20,21] and a history of previous spine surgery in four patients [15,22-24]. In 29 cases, the patient history pointed toward likely precipitating factors for OOM: head-and-neck infection in nine [3,4,6,14,25-28], seeding from other infections in nine [11,18,29-34], flu-like illness in five [14,26,35-37], iatrogenic in three (tonsillectomy and adenoidectomy [5], cervical steroid injection [16], and dental extraction [38]), meningitis in two [17,22], and trauma in one [36]. Notable misdiagnoses include one each of crowned dens syndrome [23], seronegative rheumatoid arthritis [39], and head-and-neck cancer metastasis [8].

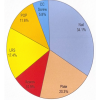
The most common presenting complaint was neck pain, observed in 47 patients (87%). Of the patients without neck pain, three were infants [40-42] and two had altered consciousness [13,17]. The next most common complaint was fever observed in 31 patients (57%), neck stiffness or decreased neck range of motion in 24 patients (44%), and torticollis or cervical/paraspinal muscle spasm in 12 patients (22%). Dysphagia or odynophagia was reported in 9 patients (17%), pharyngeal involvement (i.e. edema, erythema, or fullness) in 10 patients (19%), and cervical lymphadenopathy in 8 patients (15%). There was tenderness to palpation in the cervical or occipital region in 9 patients (17%). Neurological symptoms developed in 27 patients (50%), either at presentation or secondary to delayed response to treatment. There were 21 patients (39%) with paralysis or weakness of the extremities; of these, 9 patients (17%) developed quadriparesis. Altered mental status, including disorientation and confusion, were reported in 8 patients (15%). Leukocyte values were described for 38 patients; of these, 11 patients (29%) had normal counts of under 11,000 cells/µL. Of the remaining 27 patients (71%) with elevated leukocytes, values were provided for 22 patients and the median value was 17,700 cells/µL (range: 12,000–29,000 cells/µL). C-reactive protein (CRP) values were given for 26 patients; one patient had a normal value and the overall median was 19.8 mg/dL (range: 0.11–246 mg/dL). The results of disease processes by anatomic area as identified by radiography (XR), computed tomography (CT), or MRI are summarized in Fig. 1. XR was utilized in 24 cases (44%) and identified 8 lesions affecting the odontoid process, including erosion, destruction, and pathological fracture. Evidence of AAS or dislocation was identified in 18 patients (33%); 12 of these patients were first diagnosed through XR. CT was used for 40 patients (74%). Of these, 30 patients had evidence of OOM on CT, including erosion, bony destruction, and osteolysis. MRI was used for 46 patients (85%). A lesion affecting the odontoid process was identified in 33 patients, such as edema, abscess, lytic changes, and/or enhancement with gadolinium contrast. MRI identified almost all epidural abscesses reported.

Overall, radiologic imaging identified a process in the retropharyngeal space in 25 patients (46%), prevertebral space in 25 patients (46%), and epidural space in 19 patients (35%). Cord compression or canal narrowing was present in 20 patients (37%). The causative pathogen of the OOM was successfully identified in 38 patients (70%) and summarized in Table 2. The majority were Staphylococcus aureus (31 patients, 82%). Blood cultures were used to identify 19 cases of S. aureus infection. In 12 patients, another pathogen was identified, either in isolation or alongside S. aureus. In 9 of these patients, identification was based on culture of abscess, surgical specimen, or cerebrospinal fluid. Antibiotic therapy was pathogen-directed or broad-spectrum in cases where cultures were sterile, ranging from IV administration for 2–12 weeks with discharge antibiotics per oral for 1 week to 6 months. Twenty-two patients (41%) were treated with antibiotics alone, of which 16 (73%) made a complete recovery with no residual deficits.

There were 32 patients (59%) who underwent surgery. Debridement, drainage, or biopsy was performed in 13 patients, posterior spinal fusion in 20 patients, and spinal cord decompression in 12 patients of which 9 patients had resection of the odontoid process. Surgery for cord decompression was performed through the dorsal route for 3 patients, the transoral route for 5 patients, and the newer endonasal route in another 4 patients. All patients with decompression with the endonasal approach had odontoid resection. An external immobilization device was used in 30 patients (56%). A halo ring or halo vest was used in 14 patients, a cervical collar in 7 patients, and a Philadelphia collar in 3 patients. There were 48 patients with known follow-up of sequelae. There were 30 patients (63%) with full and complete recovery at the last follow-up. Most patients (8 of 18) with residual sequelae only describe limited cervical range of motion or stiffness. There were two deaths, due to non-treatment, noted in the two oldest articles (1944 and 1968), and one patient with residual quadriplegia and ventilator dependence, who presented in cardiopulmonary arrest [13].
Pyogenic OOM was first published in medical literature by Frank in 1944 [32]. In it, the author makes reference to the first reported case of suspected, but unconfirmed, OOM by Makins and Abbott in 1896 [43]. Since then, individual case studies have been published that describe a unique age or comorbidity of the patient, the pathogen involved, the presentation of the disease, or innovative surgical and non-surgical techniques. To our knowledge, this review of 47 articles with 54 patients is the largest to date on osteomyelitis of the odontoid process. OOM is a rare entity that requires a high degree of clinical suspicion for diagnosis. The present study seeks to highlight similarities in patient presentation to facilitate an early, accurate diagnosis of OOM and to identify best practices in its management. Pathogens may enter the vertebrae, including the odontoid process, through hematogenous spread, contiguous spread, or direct inoculation from trauma or surgery [44]. Therefore, a thorough otolaryngologic history and physical examination is a logical first step in diagnosing OOM. Factors that put a patient at risk for uncontained infection and OOM, such as advanced age, diabetes mellitus, or other causes of immunosuppression, are supported by findings in the present study. In these or other patients, a history of IV drug use, dental work, and intervention in the head-and-neck or spine including spine surgery, can expose patients to pathogens that make their way to the odontoid process [45]. Head-and-neck infections, including their sequelae of pharyngeal edema/erythema, dysphagia, or odynophagia, were also commonly reported in the present study and can be appreciated with a thorough physical exam. The classic constitutional symptoms of an infection, that is, fever, chills, and malaise, are frequently missing, complicating a successful diagnosis of OOM. Fever was inconsistently observed and only six patients had other constitutional symptoms [20,21,29,31,32,38]. Findings that may more reliably suggest OOM include neck pain and stiffness, especially pain and stiffness that is constant, prolonged, and unrelieved with analgesics. First-line investigation in the clinic should include laboratory testing; leukocytosis (71%) and elevated CRP (96%) were frequently observed, although non-specific for OOM. Imaging follows. Plain film XR was utilized in 24 (44%) of patients but only identified 8 lesions within the odontoid process. XR appears to be more valuable in identifying advanced OOM or its sequelae, including AAS or dislocation. It remains the easiest modality to administer and useful in identifying other more common pathologies of the cervical spine. Findings of the present study suggest a role for both CT and MRI in the diagnosis of OOM, although recent literature suggests MRI alone is sufficient unless contraindicated [46,47]. In both modalities, any accompanying lesions of the retropharynx, pre-vertebral space, atlantoaxial joint, or spinal cord can be appreciated. MRI has the benefit of demonstrating bone lysis earlier in the clinical course and evaluating the adjacent soft tissues, especially the epidural space; use of MRI is already recommended in cases of neck pain and fever of unknown origin [25]. MRI in OOM would show low intensity of the odontoid process on T1-weighted images and high intensity on T2-weighted images [47,48]. Cord compression and myelopathy are also better appreciated. Discussion with a neuroradiologist helps ensure the base of the skull and upper cervical spine are properly imaged to diagnose OOM and its sequelae [25]. Hematogenous spread by arterial dissemination into the odontoid process is thought to be the most likely cause for OOM due to the rich arterial supply to the odontoid process, provided by a plexus of anastomoses from branches of the vertebral arteries, ascending pharyngeal arteries, and internal carotid arteries [49]. There also exists a pharyngo-vertebral venous system that drains regions of the posterior nasopharynx, traveling inferiorly to the peri-odontoid plexus and ultimately to the upper cervical epidural plexus [49,50]. Across these two mechanisms, infection of the pharyngeal area can precipitate OOM. This is corroborated by our finding of recent head-and-neck infections in 17% of patients and distant infections in another 17% of patients. Bacteremia and infection or instrumentation in the urinary tract are known, albeit less common, risk factors for vertebral osteomyelitis. It has been reported as early as 1931 by Carson [51]. Whether local spread from an occult retropharyngeal or pre-vertebral infection causes OOM or primary OOM causes the spread of infection to the retropharyngeal and epidural spaces is subject to debate and likely differs by patient case; the latter, however, has been more commonly reported in the literature [11].
- aureus accounts for the vast majority of vertebral osteomyelitis, ranging from 40 to 80% of all cases [52]. The present study echoes these findings. It is generally agreed that the accurate identification of the offending pathogen is the first step in treatment including antibiotic susceptibility. Blood cultures successfully identified the pathogen in 39% of cases. However, other methods including urine and cerebrospinal fluid culture are useful if initial blood cultures are negative, especially given that urinary tract infections can precipitate OOM and meningitis is a common misdiagnosis, respectively [17,33]. Early transoral biopsy in settings where a retropharyngeal abscess is appreciated on physical exam is useful in that it can decompress the area, prevent further seeding of infection, and identify uncommon organisms in cases of polymicrobial infection [7,15,19]. In cases where the pathogen is identified early and there is no evidence of cervical instability, antibiotics with/without external stabilization have been demonstrated to treat the disease.
Morbidity and mortality due to OOM are largely a result of its potential to cause AAS. In this way, it is similar to other etiologies of disease affecting the odontoid process, including rheumatoid arthritis, crystal arthropathy, osteoarthritis, trauma, tumor, and congenital disease, each of which is more common than osteomyelitis and important differential diagnoses [39]. Grisel’s syndrome is another competing diagnosis and appears most commonly in children. It refers to a non-traumatic, non-infectious AAS secondary to peripharyngeal inflammation and hyperemia in the upper cervical spine [50]. Normally, a set of synovial articulations between the occiput and atlas, atlas and axis, and atlas and odontoid process are held in place by ligaments in the atlantoaxial space [34]. AAS is a result of laxity in these ligaments, most notably the transverse ligament, which causes subluxation of the atlas anterior to the axis and ultimately spinal cord compression. Most seriously this can cause paralysis of the respiratory muscles and disruption of the autonomic nervous system, but more commonly affects the descending motor tracts potentially resulting in quadriplegia [13]. Symptoms can also refer to the brainstem, cerebellum, cervical nerve roots, and lower cranial nerves [46]. This is corroborated by the present study in which 50% of patients developed neurological symptoms, 78% of whom had muscle weakness, and 33% of whom developed quadriplegia. Before 1990, AAS resulted in death for one in three patients [34]. Due to the morbidity of AAS is the complexity of the anatomy of the atlantoaxial space, making definitive radiographic diagnosis difficult [25]. An increased atlanto-dental interval (ADI) is diagnostic (ADI >3mm in adults and >5mm in children) ) and myelopathy correlates well to the degree of ADI observed on MRI [53]. In the age of regular use of MRI, coupled with increased provider awareness of OOM, medical management with antibiotics alone, with or without external cervical stabilization, is a possible and increasingly successful therapy. Sixteen of 22 patients (73%) treated medically had no long-term sequelae. However, odontoidectomy is indicated for patients with OOM that have neurologic symptoms and/or AAS and continue to decline while on antibiotics and external immobilization [24,54]. Twelve patients underwent surgery for decompression; 3 were dorsal laminectomies (1999, 2003, 2007) [29-33], 5 were transoral (2003, 2007, 2010) [14,16,55], and 4 were endoscopic endonasal (2015, 2021) [15,24]. Spinal cord decompression at the craniovertebral junction has advanced from invasive, but familiar, dorsal spine surgery at the turn of the century to less invasive means through the mouth and nose in more recent years. In the case of OOM, odontoidectomy serves to remove the collection of pathogens as well as cord decompression. Riley et al. provide a comprehensive list of common surgical approaches for odontoid resection [22]. The newer endoscopic endonasal approach for odontoidectomy and anterior decompression should be considered against the transoral route. Benefits of the endonasal approach include feasibility in the pediatric population and patients with unideal oral cavities (e.g., macroglossia, midface hypoplasia, or micrognathia) [56]. Perioperatively, there is a decreased risk of contamination by oral flora and necessary transection of the palate for surgical access. Extubation following surgery is also faster and there is a decreased risk of post-operative velopharyngeal insufficiency and dysphagia [57]. Downsides include difficulty in accessing the subaxial (C3-C7) spine; the inferior limit of the endonasal approach is predicted by the nasoaxial line, located midway of C2 [58]. If the lesion is located inferior to the nasoaxial line, the transoral route should be considered, which permits the greatest surgical bed exposure [57]. OOM is a rare entity and other etiologies of disease affecting the odontoid process are comparatively much more common. The present study seeks to provide a review of the current literature of OOM by way of previously reported case studies or series. As the previous literature is entirely composed of cases, it may be skewed toward the novel: a new risk factor, patient presentation, pathogen, sequelae, management, or surgical approach. As a result, many of the case series included other etiologies, from which osteomyelitis had to be extracted. In addition, our analysis is limited to only the information provided in the articles. For example, AAS identified by XR does not necessarily mean that AAS could not be identified on MRI; instead, it was likely just not reported. Further research may be warranted in better understanding the pathophysiology and epidemiology of OOM, especially in the current age where more frequent diagnoses of OOM are being made.
OOM is a rare entity requiring a high degree of clinical suspicion. To avoid delayed or misdiagnosis, we recommend a thorough history and physical to identify patient risk factors (advanced age, diabetes, or infection especially of the head-and-neck), salient complaints (unremitting neck pain and stiffness), and nidi of infection (retropharyngeal abscess). Leukocytosis and elevated CRP support the diagnosis, but MRI remains the gold standard. Early pathogen identification and appropriate antibiotics, with incision and drainage, when possible, can treat OOM while avoiding surgery. AAS resulting in cord compression is the sequela to avoid, at which point decompression and odontoidectomy through the endonasal route are effective and least invasive.
OOM is a rare clinical entity that should be suspected in the appropriate patient presenting with persistent neck pain progressing to extremity weakness. It is diagnosed with MRI and treated with drainage and antibiotics or decompression in cases of AAS.
References
- 1.Issa K, Diebo BG, Faloon M, Naziri Q, Pourtaheri S, Paulino CB, et al. The epidemiology of vertebral osteomyelitis in the United States from 1998 to 2013. Clin Spine Surg 2018;31:E102-8. [Google Scholar | PubMed]
- 2.Malawski SK, Lukawski S. Pyogenic infection of the spine. Clin Orthop Relat Res 1991;272:58-66. [Google Scholar | PubMed]
- 3.Uchiyama T, Akeda K, Takegami N, Takeoka M, Hirayama J, Hirayama M, et al. Osteomyelitis of the odontoid process complicated with acute mastoiditis in a 4-year-old child: Case report and literature review. JOS Case Rep 2023;2:5-8. [Google Scholar | PubMed]
- 4.Ahlbäck S, Collert S. Destruction of the odontoid process due to atlanto-axial pyogenic spondylitis. Acta Radiol Diagn (Stockh) 1970;10:394-400. [Google Scholar | PubMed]
- 5.Baker LL, Bower CM, Glasier CM. Atlantoaxial subluxation and cervical osteomyelitis: Two unusual complications of adenoidectomy. Ann Otol Rhinol Laryngol 1996;105:295-9. [Google Scholar | PubMed]
- 6.Kubo S, Takimoto H, Hosoi K, Toyota S, Karasawa J, Yoshimine T. Osteomyelitis of the odontoid process associated with meningitis and retropharyngeal abscess--case report. Neurol Med Chir (Tokyo) 2002;42:447-51. [Google Scholar | PubMed]
- 7.Tang CL, Shen CC. Nasoseptal flap revision in endoscopic endonasal odontoidectomy for acute atlantoaxial osteomyelitis with atlantoaxial subluxation. Formosan J Surg 2021;54:159-63. [Google Scholar | PubMed]
- 8.Cha JG, Hong HS, Koh YW, Kim HK, Park JM. Candida albicans osteomyelitis of the cervical spine. Skeletal Radiol 2008;37:347-50. [Google Scholar | PubMed]
- 9.Young WF, Weaver M. Isolated pyogenic osteomyelitis of the odontoid process. Scand J Infect Dis 1999;31:512-5. [Google Scholar | PubMed]
- 10.Busche M, Bastian L, Riedemann NC, Brachvogel P, Rosenthal H, Krettek C. Complete osteolysis of the dens with atlantoaxial luxation caused by infection with Staphylococcus aureus: A case report and review of the literature. Spine (Phila Pa 1976) 2005;30:E369-74. [Google Scholar | PubMed]
- 11.Rimalovski AB, Aronson SM. Abscess of medulla oblongata associated with osteomyelitis of odontoid process. Case report. J Neurosurg 1968;29:97-101. [Google Scholar | PubMed]
- 12.Kurimoto M, Endo S, Ohi M, Hirashima Y, Matsumura N, Takaku A. Pyogenic osteomyelitis of an invaginated odontoid process with rapid deterioration of high cervical myelopathy: A case report. Acta Neurochir (Wien) 1998;140:1093-4. [Google Scholar | PubMed]
- 13.Dodd KW, Weston BW, Marinelli WA, Moore JC. An unusual cause of cardiopulmonary arrest. Intern Emerg Med 2016;11:833-5. [Google Scholar | PubMed]
- 14.Suchomel P, Buchvald P, Barsa P, Lukas R, Soukup T. Pyogenic osteomyelitis of the odontoid process: Single stage decompression and fusion. Spine (Phila Pa 1976) 2003;28:E239-44. [Google Scholar | PubMed]
- 15.Burns TC, Mindea SA, Pendharkar AV, Lapustea NB, Irime I, Nayak JV. Endoscopic transnasal approach for urgent decompression of the craniocervical junction in acute skull base osteomyelitis. J Neurol Surg Rep 2015;76:e37-42. [Google Scholar | PubMed]
- 16.Reid PJ, Holman PJ. Iatrogenic pyogenic osteomyelitis of C-1 and C-2 treated with transoral decompression and delayed posterior occipitocervical arthrodesis. Case report. J Neurosurg Spine 2007;7:664-8. [Google Scholar | PubMed]
- 17.Hakeem L, Douglas JG, Laing RB. Odontoid peg and skull base osteomyelitis presenting as Streptococcus pneumoniae meningitis in diabetes mellitus. Br J Diabetes Vasc Dis 2013;13:208-10. [Google Scholar | PubMed]
- 18.Noguchi S, Yanaka K, Yamada Y, Nose T. Diagnostic pitfalls in osteomyelitis of the odontoid process: Case report. Surg Neurol 2000;53:573-8. [Google Scholar | PubMed]
- 19.Timashpolsky A, Ballard D, Sundaram K. Osteomyelitis of the odontoid process with associated retropharyngeal abscess: A case report. Otolaryngol Case Rep 2018;7:28-30. [Google Scholar | PubMed]
- 20.Venger BH, Musher DM, Brown EW, Baskin DS. Isolated C-2 osteomyelitis of hematogenous origin: Case report and literature review. Neurosurgery 1986;18:461-4. [Google Scholar | PubMed]
- 21.Keogh S, Crockard A. Staphylococcal infection of the odontoid peg. Postgrad Med J 1992;68:51-4. [Google Scholar | PubMed]
- 22.Riley K, Singh H, Meyer SA, Jenkins AL 3rd. Minimally invasive surgical approach for odontoid lesions: A technical description in a case of high cervical osteomyelitis and abscess. World Neurosurg 2016;91:332-9. [Google Scholar | PubMed]
- 23.Hashimoto E, Miyazaki K, Hirose K, Maeno T. A case of odontoid osteomyelitis. Cureus 2024;16:e52012. [Google Scholar | PubMed]
- 24.Butenschoen VM, Wostrack M, Meyer B, Gempt J. Endoscopic transnasal odontoidectomy for ventral decompression of the craniovertebral junction: Surgical technique and clinical outcome in a case series of 19 patients. Oper Neurosurg (Hagerstown) 2021;20:24-31. [Google Scholar | PubMed]
- 25.Chaudhry FB, Raza S, Ahmad U. Delayed diagnosis of odontoid peg osteomyelitis with bilateral X and XII cranial nerve palsies. BMJ Case Rep 2019;12:e227943. [Google Scholar | PubMed]
- 26.Lubotzky A, Cytter-Kuint R, Raccah E, Megged O. Osteomyelitis of the odontoid process in children: Two cases and review of the literature. Pediatr Infect Dis J 2017;36:802-5. [Google Scholar | PubMed]
- 27.Bullock R, Soares DP, James M. An infected branchial cyst complicated by retropharyngeal abscess, cervical osteomyelitis and atlanto-axial subluxation. Case Rep 2010;2010:bcr0420102933. [Google Scholar | PubMed]
- 28.Ruskin J, Shapiro S, McCombs M, Greenberg H, Helmer E. Odontoid osteomyelitis. An unusual presentation of an uncommon disease. West J Med 1992;156:306-8. [Google Scholar | PubMed]
- 29.Rajpal S, Chanbusarakum K, Deshmukh PR. Upper cervical myelopathy due to arachnoiditis and spinal cord tethering from adjacent C-2 osteomyelitis. Case report and review of the literature. J Neurosurg Spine 2007;6:64-7. [Google Scholar | PubMed]
- 30.Wiedau-Pazos M, Curio G, Grüsser C. Epidural abscess of the cervical spine with osteomyelitis of the odontoid process. Spine (Phila Pa 1976) 1999;24:133-6. [Google Scholar | PubMed]
- 31.Dimaala J, Chaljub G, Oto A, Swischuk L. Odontoid osteomyelitis masquerading as a C2 fracture in an 18-month-old male with torticollis: CT and MRI features. Emerg Radiol 2006;12:234-6. [Google Scholar | PubMed]
- 32.Frank T. Osteomyelitis of the odontoid process of the axis (dens of the epistropheus). Med J Aust 1944;1:198-201. [Google Scholar | PubMed]
- 33.Haridas A, Walsh DC, Mowle DH. Polymicrobial osteomyelitis of the odontoid process with epidural abscess: Case report and review of literature. Skull Base 2003;13:107-11. [Google Scholar | PubMed]
- 34.Yamane K, Nagashima H, Tanishima S, Teshima R. Severe rotational deformity, quadriparesis and respiratory embarrassment due to osteomyelitis at the occipito-atlantoaxial junction. J Bone Joint Surg Br 2010;92:286-8. [Google Scholar | PubMed]
- 35.Ujigo S, Kishi K, Imada H, Shibuya H, Nakanishi K, Adachi N. Upper cervical osteomyelitis with odontoid process destruction treated with a halo vest in a child: A case report. Spine Surg Relat Res 2020;4:287-9. [Google Scholar | PubMed]
- 36.Zimmermann P, Ritz N, Stranzinger E. Odontoid osteomyelitis in children: Illustrative case reports and review of the literature. Pediatr Infect Dis J 2016;35:920-3. [Google Scholar | PubMed]
- 37.Nolting L, Singer J, Hackett R, Kleiner L. Acute hematogenous osteomyelitis of the odontoid process in a child with torticollis. Pediatr Emerg Care 2010;26:669-71. [Google Scholar | PubMed]
- 38.Goes E, Zeller V, Chicheportiche V, Tristan A, Desplaces N, Ziza JM. Staphylococcus aureus osteitis of the dens: A rare location. Joint Bone Spine 2013;80:531-3. [Google Scholar | PubMed]
- 39.Sanada K, Tanaka J, Yamamoto T. Vertical subluxation caused by infection around the odontoid process: A case report. J Orthop Case Rep 2024;14:98. [Google Scholar | PubMed]
- 40.Hirsh D, Farrell K, Reilly C, Dobson S. Pasteurella multocida meningitis and cervical spine osteomyelitis in a neonate. Pediatr Infect Dis J 2004;23:1063-5. [Google Scholar | PubMed]
- 41.Papp Z, Czigléczki G, Banczerowski P. Multiple abscesses with osteomyelitis and destruction of both the atlas and the axis in a 4-week-old infant. Spine (Phila Pa 1976) 2013;38:E1228-30. [Google Scholar | PubMed]
- 42.Murray S, Coleman C, Russell-Taylor M. G452 (P) Not Just a Pain in the Neck? An Unusual Case of an Infant Presenting with a Stiff Neck, Diagnosed with Septic Arthritis of the Atlantoaxial Joint and Osteomyelitis of the Odontoid Peg. United Kingdom: BMJ Publishing Group Ltd.; 2015. [Google Scholar | PubMed]
- 43.Makins GH, Abbott FC. II. On acute primary osteomyelitis of the vertebrae. Ann Surg 1896;23:510-39. [Google Scholar | PubMed]
- 44.Berbari EF, Kanj SS, Kowalski TJ, Darouiche RO, Widmer AF, Schmitt SK, et al. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adultsa. Clin Infect Dis 2015;61:e26-46. [Google Scholar | PubMed]
- 45.Doutchi M, Seng P, Menard A, Meddeb L, Adetchessi T, Fuentes A, et al. Changing trends in the epidemiology of vertebral osteomyelitis in Marseille, France. New Microbes New Infect 2015;7:1-7. [Google Scholar | PubMed]
- 46.Jain N, Verma R, Garga UC, Baruah BP, Jain SK, Bhaskar SN. CT and MR imaging of odontoid abnormalities: A pictorial review. Indian J Radiol Imaging 2016;26:108-19. [Google Scholar | PubMed]
- 47.Zimmerli W. Vertebral osteomyelitis. N Engl J Med 2010;362:1022-9. [Google Scholar | PubMed]
- 48.Dunbar JA, Sandoe JA, Rao AS, Crimmins DW, Baig W, Rankine JJ. The MRI appearances of early vertebral osteomyelitis and discitis. Clin Radiol 2010;65:974-81. [Google Scholar | PubMed]
- 49.Gormley W, Rock J. Spontaneous atlantoaxial osteomyelitis: No longer a rare case? Case report. Neurosurgery 1994;35:132-5. [Google Scholar | PubMed]
- 50.Parke WW, Rothman RH, Brown MD. The pharyngovertebral veins: An anatomical rationale for Grisel’s syndrome. J Bone Joint Surg Am 1984;66:568-74. [Google Scholar | PubMed]
- 51.Carson HW. Acute osteomyelitis of the spine. Br J Surg 1931;18:400-8. [Google Scholar | PubMed]
- 52.Carragee EJ. Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am 1997;79:874-80. [Google Scholar | PubMed]
- 53.Yamashita Y, Takahashi M, Sakamoto Y, Kojima R. Atlantoaxial subluxation. Radiography and magnetic resonance imaging correlated to myelopathy. Acta Radiol 1989;30:135-40. [Google Scholar | PubMed]
- 54.Al-Hourani K, Al-Aref R, Mesfin A. Upper cervical epidural abscess in clinical practice: Diagnosis and management. Global Spine J 2016;6:383-93. [Google Scholar | PubMed]
- 55.Yau EL, Li KK. Concomitant fungal and bacterial atlanto-axial osteomyelitis: A case report. J Orthop Surg 2010;18:241-3. [Google Scholar | PubMed]
- 56.Hickman ZL, McDowell MM, Barton SM, Sussman ES, Grunstein E, Anderson RC. Transnasal endoscopic approach to the pediatric craniovertebral junction and rostral cervical spine: Case series and literature review. Neurosurg Focus 2013;35:E14. [Google Scholar | PubMed]
- 57.Baird CJ, Conway JE, Sciubba DM, Prevedello DM, Quiñones-Hinojosa A, Kassam AB. Radiographic and anatomic basis of endoscopic anterior craniocervical decompression: A comparison of endonasal, transoral, and transcervical approaches. Neurosurgery 2009;65:158-63. [Google Scholar | PubMed]
- 58.Aldana PR, Naseri I, La Corte E. The naso-axial line: A new method of accurately predicting the inferior limit of the endoscopic endonasal approach to the craniovertebral junction. Neurosurgery 2012;71:ons308-14; discussion ons314. [Google Scholar | PubMed]