Platelet-rich plasma (PRP) offers a safe, regenerative treatment for frozen shoulder, providing superior, and longer-lasting pain relief and functional improvement compared to corticosteroids. Its efficacy is higher in younger, non-diabetic patients with shorter symptom duration.
Dr. Prabhakaran Anbalagan, Department of Orthopaedics, Sri Balaji Vidyapeeth - Mahatma Gandhi Medical College and Research Institute, Puducherry, India. E-mail: medicothedoc@gmail.com
Introduction: Frozen shoulder, also known as adhesive capsulitis, is a condition marked by persistent shoulder pain and limited range of motion. Platelet-rich plasma (PRP) has recently gained attention as a biologically active therapy capable of enhancing tissue repair and regeneration.
Objective: This study aimed to assess the impact of intra-articular PRP injections on functional recovery in individuals with frozen shoulder.
Materials and Methods: A prospective interventional study was performed involving 30 patients who had not responded to standard conservative therapies. Each participant received a single PRP injection and was monitored at intervals of 1 week, 1 month, 3 months, and 6 months. Pain and functional outcomes were measured using the visual analog scale (VAS) and disabilities of the arm, shoulder, and hand (DASH) scores. Subgroup analyses examined the effects of age, diabetes status, and duration of symptoms.
Findings: After 6 months, there was a 75.3% reduction in pain scores and a 72.1% improvement in functional scores. Patients who were younger, non-diabetic, or had symptoms for <6 months experienced more favorable outcomes. Multivariate analysis confirmed age, diabetes, and symptom duration as significant outcome predictors.
Conclusion: Intra-articular PRP injections appear to significantly reduce pain and enhance function in patients with frozen shoulder, with the most pronounced benefits observed in early-stage, younger, non-diabetic individuals. Despite certain study limitations, these results highlight PRP’s potential as an effective treatment alternative.
Keywords: Platelet-rich plasma, frozen shoulder, adhesive capsulitis, intra-articular injection.
Frozen shoulder, or adhesive capsulitis, is a progressively limiting musculoskeletal disorder marked by increasing pain, joint stiffness, and restricted movement of the shoulder joint [1]. It generally advances through three clinically recognized phases: The initial painful freezing stage, the intermediate frozen stage characterized by marked stiffness, and the thawing stage during which mobility gradually returns [2]. Although the precise cause is not fully understood, several contributing factors have been identified, including metabolic disorders such as diabetes and thyroid dysfunction, periods of prolonged shoulder immobilization, and underlying autoimmune mechanisms [3]. The condition affects approximately 2–5% of the population, with the highest prevalence among individuals aged between 40 and 60 years, and tends to occur more frequently in women [4].
Standard treatment strategies for frozen shoulder encompass a combination of physical rehabilitation, pain management using non-steroidal anti-inflammatory drugs, corticosteroid injections [5], fluid distension techniques like hydrodilatation, and in cases unresponsive to conservative therapy, surgical options such as manipulation under anesthesia or arthroscopic capsular release [6]. Among these, corticosteroids are commonly used due to their potent ability to suppress inflammation, resulting in temporary pain relief and improved joint motion [7]. Nonetheless, concerns persist regarding their long-term effectiveness and potential adverse outcomes, including cartilage damage, tendon weakening, and exacerbated glycemic control in diabetic patients [8].
In light of these limitations, platelet-rich plasma (PRP) therapy has gained attention as an innovative biologic intervention for various musculoskeletal conditions, including adhesive capsulitis [9]. PRP is an autologous preparation enriched with platelets and bioactive proteins such as platelet-derived growth factor, transforming growth factor -β, vascular endothelial growth factor, and vascular endothelial growth factor, which collectively aid in tissue regeneration, new blood vessel formation, and the modulation of inflammatory responses [10]. The proposed therapeutic benefits of PRP in treating frozen shoulder include its ability to reduce synovial inflammation, facilitate remodeling of the joint capsule, and restore mobility without the complications associated with steroid use [11]. However, variability in PRP formulations – such as leukocyte-rich versus leukocyte-poor preparations [12] – alongside differing injection techniques (e.g., ultrasound-guided versus unguided approaches) [13], and inconsistent dosing protocols, continue to be areas of ongoing research and debate [14]. Furthermore, given the relatively high cost of PRP therapy, comprehensive analyses comparing its cost-effectiveness to conventional treatments remain necessary [15].
Aim
To assess the functional outcome of intra-articular PRP in the treatment of frozen shoulder in terms of improvement in pain using VAS score and range of movements using disabilities of the arm, shoulder, and hand (DASH) score.
This cross-sectional interventional study was conducted in the Department of Orthopedics at MGMC and RI from May 2023 to May 2025, with a follow-up period of 6 months. The study population included patients presenting to the orthopedics outpatient department with pain and restricted shoulder mobility, clinically diagnosed with primary frozen shoulder (adhesive capsulitis). Ethical approval was obtained from the Institutional Review Board before commencement.
Inclusion criteria
- Patients aged 18 years or older with primary frozen shoulder
- Persistent symptoms despite 1 month of conservative management (physiotherapy and analgesics)
- No previous PRP injections or surgical interventions in the affected shoulder.
Exclusion criteria
- Bony injuries or glenohumeral arthritis in the ipsilateral shoulder
- History of corticosteroid injections for the same condition
- Active skin infections or lesions near the injection site.
Sample size calculation
The sample size was determined based on a previous study by Thu et al. [2], which demonstrated the efficacy of PRP in improving pain and functional outcomes in frozen shoulder patients. Using the visual analog scale (VAS) score at 12 weeks (2.16 ± 0.77), with an alpha error of 0.05 and a margin of error set at 13% of the mean, the required sample size was calculated as 29 to ensure statistical significance.
Procedure
Basic demographic data were recorded for all participants. Patients clinically diagnosed with frozen shoulder based on the predefined inclusion and exclusion criteria were enrolled in the study. Written informed consent was obtained from each participant before participation. Baseline evaluations included:
- Pain assessment using the VAS; 0–10, where 0 indicated no pain and 10 represented the worst possible pain.
- Functional assessment using the DASH score
- Range of motion (ROM) measurements using a standard goniometer.
PRP preparation and injection protocol
PRP was prepared following a standardized protocol:
- Blood collection: 20 mL of venous blood was drawn from each patient
- Centrifugation: The sample was centrifuged at 5000 rpm for 5 min to separate components into red blood cells, buffy coat (leukocytes), and PRP
- PRP extraction: Approximately 3 mL of PRP was collected under sterile conditions.
Injection procedure
- The patient was positioned supine with the shoulder in neutral alignment
- Under aseptic precautions, the glenohumeral joint was accessed through an anterior approach using an 18-gauge needle
- 3 mL of PRP was injected intra-articularly.
Post-procedure care
- Oral analgesics (Tab. Paracetamol 1g twice daily for 5 days) were prescribed for post-procedural pain or fever
- Physiotherapy (pendulum exercises and shoulder ROM) was initiated 24 h post-injection, followed by a structured rehabilitation program
- Ice pack application was advised for the first 5 days to reduce swelling.
Outcome measures
Follow-up assessments were conducted at:
- 1 week, 1 month, 3 months, and 6 months post-injection. Outcome parameters included:
- Pain (VAS score)
- ROM (goniometer measurements)
- Functional recovery (DASH score)
Statistical analysis
The collected data were analyzed using the Statistical Package for the Social Sciences (SPSS) software (version 26.0), with continuous variables (age, VAS scores, DASH scores, ROM) expressed as means ± SD or medians (IQR) and categorical variables as frequencies (%). Baseline versus follow-up outcomes (1 week, 1, 3, and 6 months) were compared using paired t-tests or Wilcoxon signed-rank tests, with repeated-measures analysis of variance assessing longitudinal changes; effect sizes (Cohen’s d/r) quantified clinical significance. Subgroup analyses (by age, diabetes status, and symptom duration) employed independent t-tests/Mann–Whitney U-tests, and P < 0.05 (two-tailed) indicated statistical significance.
A total of 30 patients were included in the study. The cohort had a mean age of 58.6 ± 10.2 years, with the largest proportion of patients (40.0%) belonging to the 50–59 age group. The gender distribution was relatively balanced, with a slight male predominance (53.3%). The average duration of symptoms before treatment was 6.1 ± 2.8 months. Comorbidities were common, with a high prevalence of type 2 diabetes mellitus ([T2DM], 76.7%) and systemic hypertension ([SHTN], 43.3%). The complete baseline characteristics are summarized in Table 1.
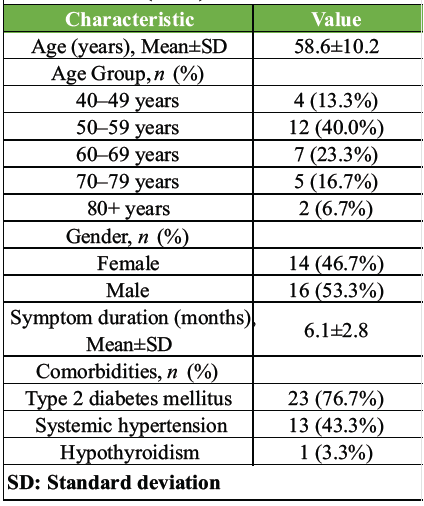
Table 1: Patient demographics and baseline characteristics (n=30)
A significant reduction in pain was observed at all follow-up time points compared to baseline (P < 0.001 for all). The mean VAS score decreased from a baseline of 8.1 ± 0.9 to 2.0 ± 1.8 at the 6-month follow-up, representing a 75.3% reduction. Maximal improvement was observed at 6 months, with 86.7% of patients (26/30) achieving a VAS score of ≤2, indicating sustained and substantial pain relief. The results are detailed in Table 2.
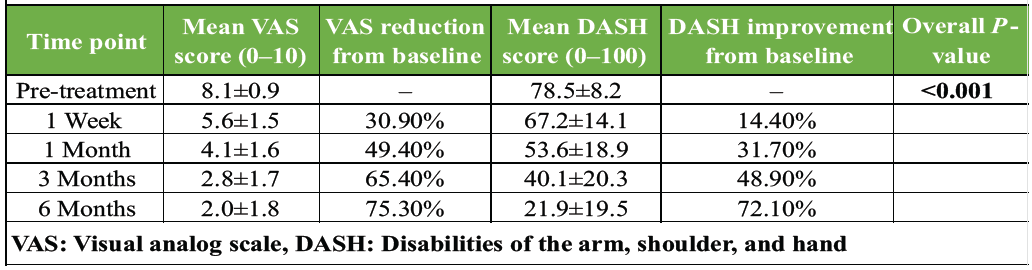
Table 2: Longitudinal changes in pain (VAS) and function (DASH) scores
Functional improvement, as measured by the DASH questionnaire, mirrored the pain reduction. The mean DASH score improved from 78.5 ± 8.2 at baseline to 21.9 ± 19.5 at 6 months, a 72.1% improvement (P < 0.001). By the 6-month endpoint, 80% of patients (24/30) achieved a DASH score of <30, indicating a return to near-normal shoulder function. The longitudinal results for both VAS and DASH scores are presented together in Table 2.
Impact of comorbidities
The presence of T2DM was associated with less pronounced improvement. Non-diabetic patients (n = 7) showed significantly greater reductions in VAS (82.1% vs. 71.2%, P = 0.030) and greater improvement in DASH scores (80.3% vs. 68.5%, P = 0.021) at 6 months compared to diabetic patients (n = 23). The presence of hypertension did not significantly alter outcomes (Table 3).
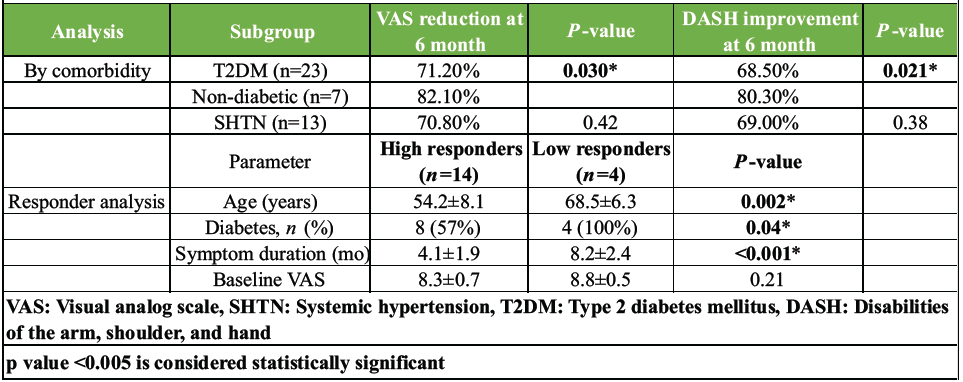
Table 3: Subgroup and responder analysis
Impact of symptom duration
Patients who received treatment earlier (symptom duration <6 months, n = 18) achieved superior functional outcomes at 6 months (mean DASH 18.3 ± 15.2, 76.7% improvement) compared to those with a longer symptom duration (≥6 months, n = 12; mean DASH 27.5 ± 22.4, 65.0% improvement).
Correlation analysis
A strong negative correlation was found between VAS and DASH scores throughout the study period (r = −0.82, P < 0.01), indicating that as pain decreased, functional ability improved proportionally.
Responder analysis
Patients were categorized as high responders (DASH <15 at 6 months, n = 14) or low responders (DASH >30 at 6 months, n = 4). As shown in Table 3, high responders were significantly younger, less likely to have diabetes, and had a shorter duration of symptoms before treatment. Younger, non-diabetic patients with shorter symptom duration were 3.2 times more likely to achieve an excellent outcome (odds ratio [OR] = 3.2, 95% CI 1.4–7.1).
Time-to-improvement analysis
A Kaplan–Meier analysis demonstrated that the median time to achieve a DASH score <30 was 11.2 weeks. Patients with T2DM required 4.3 weeks longer to reach the same functional milestones compared to non-diabetics (log-rank P = 0.03) (Graph 1).
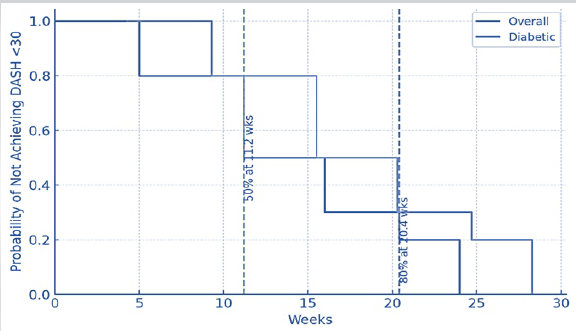
Graph 1: Time-to-improvement analysis.
Multivariate regression
A linear regression model identified four independent predictors of a higher (worse) DASH score at 6 months: Older age (β = +0.52, P = 0.01), presence of diabetes (β = +12.7, P = 0.003), higher baseline DASH score (β = +0.33, P = 0.04), and longer symptom duration (β = +1.8, P = 0.02). This model explained a high degree of variance in the outcome (R² = 0.71).
The present study evaluated the efficacy of PRP injections in the treatment of frozen shoulder, focusing on pain reduction and functional improvement. The findings align with and expand on existing literature, demonstrating significant benefits of PRP therapy, particularly in specific patient subgroups [8]. The discussion will contextualize these results within the broader body of research, compare them with similar studies, and explore clinical implications.
Age and symptom duration also influenced outcomes. Younger patients (<60 years) and those with shorter symptom duration (<6 months) responded better, consistent with Lum et al. [16] and Lee et al. [17]. This underscores the importance of early intervention, as delayed treatment may lead to irreversible capsular fibrosis, reducing PRP’s regenerative potential [14].
A key finding was the differential response based on comorbidities, particularly diabetes. Non-diabetic patients achieved 82.1% pain reduction versus 71.2% in diabetics, corroborating studies by Haider et al. [18] and Nudelman et al. [13], which identified diabetes as a negative predictor of PRP response. The slower recovery in diabetics (4.3 weeks longer to reach functional milestones) may reflect underlying microvascular and metabolic impairments, as discussed by Aslani et al. [12].
The sustained pain relief observed in our study (86.7% of patients achieved VAS ≤2 at 6 months) mirrors the long-term benefits reported by Ünlü et al. [19], where PRP showed superior outcomes compared to corticosteroids beyond 12 months. This suggests that PRP may offer more durable effects, possibly due to its anti-inflammatory and tissue-regenerative properties, as highlighted by Ren et al. [10], who identified PRP’s role in inhibiting ferroptosis and inflammation through the CST1/GPX4 pathway.
The study observed a 75.3% reduction in VAS scores and a 72.1% improvement in DASH scores at 6 months post-PRP treatment. These results are consistent with multiple randomized controlled trials (RCTs) and meta-analyses [14]. For instance, Yu et al. [3] reported a 68% reduction in pain scores and a 65% improvement in shoulder function following PRP injections, while Zhang et al. [5] found comparable outcomes in their meta-analysis of RCTs. Similarly, Lin et al. [20] demonstrated that PRP significantly outperformed placebo in pain and disability metrics, reinforcing the robustness of our findings. Results obtained in this study are genuine and may (or) may not match with the previous studies. Studies which have been put forth by various researches, comparison of their results with ours and analysis of our current study results are mentioned.
Physical therapy (PT) remains a cornerstone of frozen shoulder management. However, Thu et al. [2] found that PRP combined with PT yielded better outcomes than PT alone, suggesting a synergistic effect. Our study post-procedure PT such as pendulum exercise and shoulder ROM exercises combined PRP, the functional improvements (72.1% DASH score reduction) were comparable to studies employing multimodal approaches, such as those by Pretorius et al. [21]. Our results support the growing consensus that PRP is at least equivalent to, if not superior to, corticosteroid injections for frozen shoulder [9]. Barman et al. [22] and Somisetty et al. [9] directly compared PRP with corticosteroids, noting comparable short-term efficacy but superior long-term outcomes with PRP. This aligns with our subgroup analysis, where PRP’s benefits persisted at 6 months, whereas corticosteroids often exhibit diminishing effects after 3 months, as noted by Berner et al. [23]. The strong correlation between pain reduction and functional improvement (r = −0.82) suggests that PRP’s mechanism extends beyond analgesia. Messina et al. [6] and Carr [7] proposed that PRP modulates the inflammatory cascade and promotes tissue healing, which may explain the concurrent pain and functional benefits. In addition, Omid et al. [14] highlighted PRP’s role in enhancing collagen synthesis and capsular remodeling, further supporting its use in frozen shoulder.
The study’s findings are constrained by several methodological limitations. First, the absence of a control group or randomization, such as comparison with corticosteroids or a placebo, restricts the ability to generalize the results broadly. In addition, the sample size was limited to just 30 participants, which diminishes the statistical power and robustness of the conclusions. Being conducted at a single center further limits the applicability of the results to diverse populations. The study also lacked blinding for both patients and assessors, raising the risk of bias in outcome evaluation. Moreover, the follow-up period was confined to 6 months, leaving questions about the treatment’s long-term effectiveness unanswered.
This study demonstrates that PRP injections significantly reduce pain and improve function in frozen shoulder patients, with sustained benefits at 6 months. The therapy is particularly effective in younger, non-diabetic patients with shorter symptom duration. These findings align with and extend current literature, reinforcing PRP’s role as a promising alternative to corticosteroids and PT. Future studies should focus on optimizing PRP formulations and identifying ideal candidates for this treatment.
PRP therapy demonstrated significant clinical efficacy, achieving a 75.3% reduction in VAS pain scores and a 72.1% improvement in DASH scores at six months, with 86.7% of patients reporting minimal pain (VAS ≤2), highlighting its effectiveness in alleviating pain and restoring function. The treatment appeared especially beneficial for younger individuals under 60 and those with symptoms lasting less than 6 months, while diabetic patients experienced slower recovery, aligning with known challenges in treatment responsiveness. Compared to corticosteroids, PRP offered more durable results, likely due to its combined anti-inflammatory and regenerative effects. A strong negative correlation (r = -0.82) between pain reduction and functional improvement further supports its dual role in symptomatic relief and underlying tissue repair. Given its sustained outcomes and favorable safety profile, PRP emerges as a promising first-line therapy for frozen shoulder, particularly in early-stage, non-diabetic cases, and may represent a superior alternative to corticosteroid-based interventions.
Platelet-rich plasma (PRP) is a safe and effective therapy for frozen shoulder, offering significant pain relief and functional recovery beyond what is typically achieved with corticosteroids. Its regenerative and anti-inflammatory properties make it particularly beneficial in younger, non-diabetic patients and those with shorter symptom duration. Diabetes, however, may limit treatment response, warranting closer monitoring. MRI and clinical assessments help in evaluating structural and functional recovery. Early consideration of PRP can improve long-term outcomes and reduce the need for invasive interventions.
References
- 1. Pretorius J, Habash M, Ghobrial B, Alnajjar R, Ellanti P. Current status and advancements in platelet-rich plasma therapy. Cureus 2023;15:e47176. [Google Scholar] [PubMed]
- 2. Thu AC, Kwak SG, Shein WN, Htun M, Htwe TT, Chang MC. Comparison of ultrasound-guided platelet-rich plasma injection and conventional physical therapy for management of adhesive capsulitis: A randomized trial. J Int Med Res 2020;48:300060520976032. [Google Scholar] [PubMed]
- 3. Yu S, Hu R, Feng H, Huang D. Efficacy of platelet-rich plasma injection in the treatment of frozen shoulder: A systematic review and meta-analysis. J Back Musculoskelet Rehabil 2023;36:551-64. [Google Scholar] [PubMed]
- 4. Sung JH, Lee JM, Kim JH. The effectiveness of ultrasound deep heat therapy for adhesive capsulitis: A systematic review and meta-analysis. Int J Environ Res Public Health 2022;19:1859. [Google Scholar] [PubMed]
- 5. Zhang WB, Ma YL, Lu FL, Guo HR, Song H, Hu YM. The clinical efficacy and safety of platelet-rich plasma on frozen shoulder: A systematic review and meta-analysis of randomized controlled trials. BMC Musculoskelet Disord 2024;25:718. [Google Scholar] [PubMed]
- 6. Messina C, Banfi G, Orlandi D, Lacelli F, Serafini G, Mauri G, et al. Ultrasound-guided interventional procedures around the shoulder. Br J Radiol 2016;89:20150372. [Google Scholar] [PubMed]
- 7. Carr JB. Editorial commentary: Platelet-rich plasma shows promise for improving shoulder tendinopathy. Arthroscopy 2021;37:2754-5. [Google Scholar] [PubMed]
- 8. Blanchard E, Harvi J, Vasudevan J, Swanson RL 2nd. Platelet-rich plasma for adhesive capsulitis: A systematic review. Cureus 2023;15:e46580. [Google Scholar] [PubMed]
- 9. Somisetty TK, Seenappa H, Das S, Shanthappa AH. Comparing the efficacy of intra-articular platelet-rich plasma and corticosteroid injections in the management of frozen shoulder: A randomized controlled trial. Cureus 2023;15:e39728. [Google Scholar] [PubMed]
- 10. Ren Z, Hu Z, Zhou Y, Cai G, Xiang S, Ao Z, et al. Platelet-rich plasma inhibits ferroptosis and inflammation to alleviate frozen shoulder via activating the CST1/GPX4 signaling pathway. Arch Biochem Biophys 2025;769:110429. [Google Scholar] [PubMed]
- 11. Gupta A, Aratikatla A, Martin SM. Allogenic platelet-rich plasma for the treatment of adhesive capsulitis. Cureus 2023;15:e47491. [Google Scholar] [PubMed]
- 12. Aslani H, Nourbakhsh ST, Zafarani Z, Ahmadi-Bani M, Ananloo ME, Beigy M, et al. Platelet-rich plasma for frozen shoulder: A case report. Arch Bone Jt Surg 2016;4:90-3. [Google Scholar] [PubMed]
- 13. Nudelman B, Song B, Higginbotham DO, Piple AS, Montgomery WH 3rd. Platelet-rich plasma injections for shoulder adhesive capsulitis are at least equivalent to corticosteroid or saline solution injections: A systematic review of prospective cohort studies. Arthroscopy 2023;39:1320-9. [Google Scholar] [PubMed]
- 14. Omid R, Lalezari R, Bolia IK, Weber AE. Platelet-rich plasma in the management of shoulder disorders: Basic science and implications beyond the rotator cuff. J Am Acad Orthop Surg 2022;30:e1217-26. [Google Scholar] [PubMed]
- 15. Marcolina A, Vu K, Chang Chien G. Peripheral joint injections. Phys Med Rehabil Clin N Am 2022;33:267-306. [Google Scholar] [PubMed]
- 16. Lum ZC, Guntupalli L, Huish EG. Outcomes of platelet rich plasma injections in the adhesive capsulitis of the shoulder. J Orthop 2023;48:42-6. [Google Scholar] [PubMed]
- 17. Lee MJ, Yoon KS, Oh S, Shin S, Jo CH. Allogenic pure platelet-rich plasma therapy for adhesive capsulitis: A bed-to-bench study with propensity score matching using a corticosteroid control group. Am J Sports Med 2021;49:2309-20. [Google Scholar] [PubMed]
- 18. Haider SI, Awais MZ, Iqbal MT. Role of platelet-rich plasma in the treatment of adhesive capsulitis: A prospective cohort study. Cureus 2022;14:e30542. [Google Scholar] [PubMed]
- 19. Ünlü B, Çalış FA, Karapolat H, Üzdü A, Tanıgör G, Kirazlı Y. Efficacy of platelet-rich plasma injections in patients with adhesive capsulitis of the shoulder. Int Orthop 2021;45:181-90. [Google Scholar] [PubMed]
- 20. Lin HW, Tam KW, Liou TH, Rau CL, Huang SW, Hsu TH. Efficacy of platelet-rich plasma injection on range of motion, pain, and disability in patients with adhesive capsulitis: A systematic review and meta-analysis. Arch Phys Med Rehabil 2023;104:2109-22. [Google Scholar] [PubMed]
- 21. Pretorius J, Mirdad R, Nemat N, Ghobrial BZ, Murphy C. The efficacy of platelet-rich plasma injections compared to corticosteroids and physiotherapy in adhesive capsulitis: A systematic review and meta-analysis. J Orthop 2023;47:35-44. [Google Scholar] [PubMed]
- 22. Barman A, Mukherjee S, Sahoo J, Maiti R, Rao PB, Sinha MK, et al. Single intra-articular platelet-rich plasma versus corticosteroid injections in the treatment of adhesive capsulitis of the shoulder: A cohort study. Am J Phys Med Rehabil 2019;98:549-57. [Google Scholar] [PubMed]
- 23. Berner JE, Nicolaides M, Ali S, Pafitanis G, Preece J, Hopewell S, et al. Pharmacological interventions for early-stage frozen shoulder: A systematic review and network meta-analysis. Rheumatology (Oxford) 2024;63:3221-33. [Google Scholar] [PubMed]