Advanced quantitative MRI techniques (T2 and T1ρ mapping) reliably reflect early biochemical cartilage degeneration and moderately correlate with subchondral bone changes, establishing MRI as a sensitive, non-invasive tool for early diagnosis and monitoring of knee osteoarthritis.
Dr. Akash Pradip Bera, Department of Orthopaedics, Gauri Devi Institute of Medical Sciences and Hospital, Rajbandh, West Bengal, India. E-mail: bera.akash.ab@gmail.com
Introduction: The study aimed to investigate the correlation between advanced magnetic resonance imaging (MRI) parameters of articular cartilage and subchondral bone with histopathological changes in patients with early knee osteoarthritis (OA), to assess the utility of MRI in detecting early degenerative changes.
Materials and Methods: A prospective observational study was conducted in 40 patients aged 40–65 years with clinical and radiographic evidence of early knee OA (Kellgren–Lawrence grade I–II). All patients underwent 3.0 Tesla MRI, including morphological sequences, T2 mapping, and T1ρ mapping, to evaluate cartilage integrity and subchondral bone lesions. Cartilage and subchondral bone samples were obtained during arthroscopy or planned surgical procedures and analyzed histologically using Hematoxylin and Eosin and Safranin-O/Fast Green staining. Cartilage degeneration was graded using the Mankin scoring system, and subchondral bone changes were assessed for marrow edema, trabecular remodeling, and fibrosis. MRI parameters were correlated with histopathological scores, and statistical analysis was performed using Pearson’s/Spearman’s correlation and Cohen’s kappa statistics.
Results: MRI detected early cartilage degeneration, predominantly in the medial femoral condyle and tibial plateau, with elevated T2 (42.8 ± 6.2 ms) and T1ρ (48.6 ± 7.1 ms) values in affected regions. Histopathology revealed early degenerative changes with mean Mankin scores of 4.2 ± 1.5 and subchondral bone alterations, including marrow edema (40%), trabecular remodeling (30%), and fibrosis (25%). A strong positive correlation was observed between T2/T1ρ relaxation times and Mankin scores (r = 0.72 and r = 0.69, respectively; P < 0.001). Semiquantitative MRI assessment of bone marrow lesions showed moderate agreement with histopathology (Cohen’s kappa = 0.61).
Conclusion: Advanced MRI techniques, including T2 and T1ρ mapping, accurately reflect early cartilage degeneration and show moderate correlation with subchondral bone changes. These findings support the use of MRI as a non-invasive diagnostic tool for early detection and assessment of knee OA, particularly in the medial compartment. However, the findings should be interpreted with caution due to the small sample size and the cross-sectional design of the study.
Keywords: Early knee osteoarthritis, magnetic resonance imaging, T2 mapping, T1ρ mapping, cartilage degeneration, subchondral bone, histopathology, mankin score.
Osteoarthritis (OA) of the knee is a prevalent, progressive joint disorder characterized by degeneration of articular cartilage and concomitant changes in subchondral bone, synovium, and peri-articular tissues [1]. Early-stage disease, when structural damage may still be limited and potentially modifiable is the critical window for interventions that could alter disease trajectory and preserve function [2]. Recent work has emphasized that OA is a whole-organ disease rather than an isolated cartilage disorder; subchondral bone alterations (including bone marrow lesions (BMLs) and abnormal remodeling) frequently accompany, and in some cases precede, cartilage degeneration and may contribute directly to pain and progression [3,4].
Magnetic resonance imaging (MRI) has become the imaging modality of choice for comprehensive, non-invasive assessment of knee OA because it visualizes all joint tissues (cartilage, bone, meniscus, synovium, and ligaments) and can detect early structural and compositional changes not visible on radiographs [5,6]. Advanced MRI techniques – including compositional sequences (T1ρ, T2, T2*, and quantitative mapping), high-resolution morphological sequences, and contrast-enhanced protocols – allow assessment of cartilage matrix composition (proteoglycan and collagen integrity), cartilage morphology, and subchondral bone abnormalities, such as marrow lesions and cysts [7,8]. These MRI approaches offer the potential to detect biochemical and microstructural changes that precede frank surface loss [9].
Although multiple studies have demonstrated that quantitative MRI metrics (for example, increased T1ρ and T2 relaxation times) are associated with biochemical matrix changes and with clinical or radiographic OA grades, direct clinicopathologic validation – correlating in vivo MRI findings with histopathological assessment of the same tissue regions – remains limited, especially in early OA [10,11]. Several investigations using surgical or ex vivo cartilage samples have shown moderate-to-strong correlations between relaxometry values and histological scores of collagen disruption and proteoglycan loss, while other studies highlight variability related to imaging technique, tissue sampling, loading conditions, and spatial registration between imaging and histology [2,5]. Similarly, MRI-detected subchondral BMLs and microarchitectural alterations have been linked to histological evidence of marrow edema, fibrosis, and increased remodeling activity, but precise MRI–histopathology mapping in early disease is not yet well established [6].
A robust, region-matched MRI–histopathology correlation in patients with early knee OA would strengthen the biological interpretation of MRI biomarkers, clarify which imaging features best reflect early cartilage and bone pathology, and inform the use of MRI for diagnosis, prognosis, and monitoring of disease-modifying therapies [7]. This study was therefore designed to perform systematic, spatially registered comparisons between preoperative MRI (morphological and compositional sequences) and histopathology from arthroscopic/biopsy samples obtained from patients with symptomatic early knee OA. Our primary objective was to determine whether specific MRI markers of cartilage and subchondral bone reliably correlate with histopathological grades of cartilage degeneration and bone marrow pathology. We hypothesized that quantitative compositional MRI measures (e.g., elevated T1ρ/T2/T2* values) and focal morphological findings (cartilage surface irregularity, focal thinning) would correlate with histological evidence of proteoglycan loss, collagen disruption, and early cartilage fibrillation, and that MRI-detected BMLs would correspond to histological indicators of marrow edema and bone remodeling.
Study design
This study was a multicentric study conducted in the Departments of Orthopaedics, Radiodiagnosis, and Pathology. The primary aim of the study was to evaluate the correlation between MRI findings of articular cartilage and subchondral bone with histopathological changes in patients diagnosed with early knee OA.
Patient population
Patients aged 40–65 years presenting with clinical symptoms of knee pain and stiffness suggestive of early OA were screened for inclusion in the study. Radiographic evaluation using the Kellgren–Lawrence grading system confirmed early OA (grades I or II). Patients were included if they were scheduled for diagnostic or therapeutic arthroscopy/arthroplasty and consented to tissue sampling, and if they were eligible for MRI without contraindications, such as pacemakers, metallic implants, or severe claustrophobia. Patients with advanced OA (grades III–IV), prior knee surgery or trauma, inflammatory arthritis, metabolic bone disease, or poor-quality MRI scans due to motion or technical artifacts were excluded. As this was an exploratory study with strict eligibility criteria, the final sample size remained limited, which may affect the statistical power and generalizability of the results. A total of 30 patients meeting these criteria were enrolled consecutively. Demographic details, including age, sex, body mass index (BMI), symptom duration, and the affected knee, were systematically recorded.
MRI protocol
All MRI examinations were performed using a 3.0 Tesla scanner (Philips/Siemens/GE) with a dedicated knee coil. The imaging protocol consisted of morphological, compositional, and additional sequences. Morphological sequences included proton density–weighted fat-suppressed images acquired in sagittal, coronal, and axial planes to evaluate cartilage surface integrity and subchondral bone structure. Compositional imaging included T2 mapping sequences for assessment of collagen network organization and water content, and T1ρ mapping for evaluating proteoglycan integrity. Additional sequences, including T1-weighted and Short Tau Inversion Recovery (STIR), were used for the detection and characterization of BMLs. Cartilage was systematically assessed in six anatomical regions: Medial femoral condyle, lateral femoral condyle, medial tibial plateau, lateral tibial plateau, patella, and trochlea. Subchondral bone lesions were scored semiquantitatively to allow correlation with histopathological findings.
Arthroscopic tissue sampling
During arthroscopy or planned surgical interventions, cartilage and subchondral bone specimens were collected from anatomical regions corresponding to the MRI-identified areas. To ensure accurate spatial correlation between imaging and histology, intraoperative photographs and mapping of biopsy sites to MRI slices were obtained. Specimens were immediately fixed in 10% neutral buffered formalin to preserve tissue morphology for histopathological analysis. Despite careful intraoperative mapping, minor spatial mismatches between MRI slices and biopsy sites may have occurred due to cartilage curvature, slice thickness, and tissue handling.
Histopathological examination
Collected specimens were processed for histological evaluation using standard techniques. Decalcification was performed where necessary, followed by paraffin embedding and sectioning at 4 µm thickness. Sections were stained with Hematoxylin and Eosin (H&E) to assess tissue structure and cellularity, and Safranin-O/Fast Green to evaluate proteoglycan content. Cartilage was graded according to the Mankin scoring system, which considers structure, cellularity, and proteoglycan depletion. Subchondral bone was evaluated for marrow edema, trabecular remodeling, and fibrosis. All histopathological assessments were conducted independently by two pathologists blinded to MRI findings, ensuring unbiased evaluation of the samples. Standard processing steps, such as fixation, decalcification, and sectioning may introduce microscopic artifacts that could influence morphological interpretation and histological grading.
MRI–histopathology correlation
MRI parameters, including morphological cartilage changes, T1ρ/T2 relaxation times, and subchondral BMLs, were correlated region-wise with histopathological scores. Both quantitative measures (continuous T1ρ/T2 relaxation times) and semiquantitative scores (ordinal grading of cartilage and bone changes) were analyzed. This approach allowed precise evaluation of the concordance between imaging findings and histopathological changes in early OA.
Statistical analysis
All statistical analyses were performed using Statistical Package for the Social Sciences version 25.0 (IBM Corp., Armonk, NY, USA). Continuous variables were expressed as mean ± standard deviation, while categorical variables were described using frequencies and percentages. Correlation between MRI parameters and histopathological scores was assessed using Pearson’s or Spearman’s correlation coefficients, depending on data distribution. Agreement between categorical MRI and histological grades was evaluated using Cohen’s kappa statistic. A P < 0.05 was considered statistically significant for all analyses.
Ethical considerations
Ethical approval was obtained from the Institutional Ethics Committee, and written informed consent was secured from all participants before enrollment.
Patient demographics
A total of 40 patients with early knee OA were included in the study. The study cohort comprised 22 males (55%) and 18 females (45%), with a mean age of 54.2 ± 6.1 years (range 42–65 years). The mean BMI was 27.4 ± 3.2 kg/m², and the average duration of knee symptoms was 14.6 ± 5.8 months. The right knee was affected in 24 patients (60%), while the left knee was involved in 16 patients (40%). All participants underwent MRI and arthroscopic tissue sampling without complications. Potential confounding factors, such as BMI, limb alignment, metabolic comorbidities, and physical activity levels were not controlled and may have influenced cartilage and subchondral bone characteristics.
MRI findings
Cartilage morphology
Morphological MRI sequences revealed early cartilage degeneration predominantly in the medial femoral condyle and medial tibial plateau. Focal thinning and surface irregularities were noted in 55% and 50% of patients, respectively. Less frequent involvement was observed in the lateral femoral condyle (20%), lateral tibial plateau (18%), patella (12%), and trochlea (15%).
Cartilage compositional imaging
T2 mapping demonstrated prolonged relaxation times in degenerated cartilage regions, indicative of collagen network disruption and increased water content. The mean T2 relaxation time in affected regions was 42.8 ± 6.2 ms, compared to 33.4 ± 4.1 ms in normal-appearing cartilage. T1ρ mapping revealed a mean value of 48.6 ± 7.1 ms in degenerated cartilage versus 35.7 ± 5.0 ms in healthy cartilage, reflecting early proteoglycan depletion (Table 1 and Fig. 1).
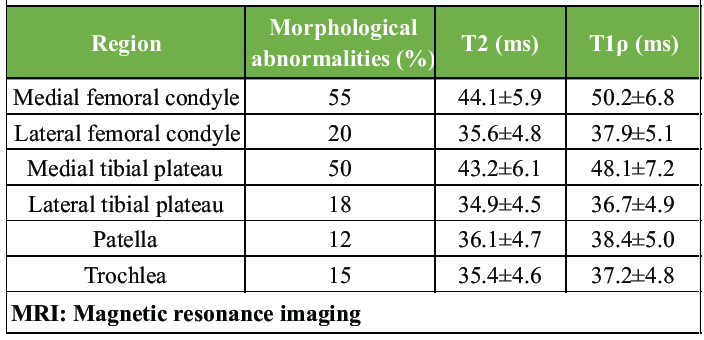
Table 1: MRI cartilage parameters
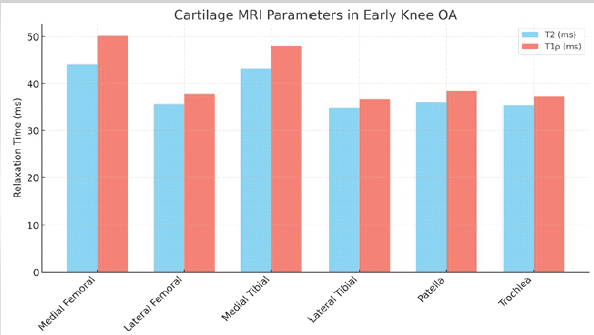
Figure 1: Magnetic resonance imaging cartilage parameters.
Subchondral bone
MRI evaluation identified BMLs in 45% of patients, predominantly in the medial femoral condyle and tibial plateau. Most lesions were small (<10 mm, 70%), while the remainder were moderate (10–20 mm, 30%). STIR sequences demonstrated hyperintense signals consistent with marrow edema.
Histopathological findings
Cartilage
Histopathological analysis confirmed early degenerative changes. The mean Mankin score across all regions was 4.2 ± 1.5. The medial femoral condyle and medial tibial plateau demonstrated the highest degeneration with Mankin scores of 4.8 ± 1.4 and 4.5 ± 1.3, respectively. Observed changes included surface fibrillation, proteoglycan loss, and occasional chondrocyte clustering. Hypocellularity was observed in 20% of samples.
Subchondral bone
Evaluation of subchondral bone revealed marrow edema in 40% of samples, trabecular remodeling in 30%, and fibrosis in 25%. Mild osteoid deposition was noted in 15% of specimens. These changes were most pronounced in regions corresponding to MRI-identified BMLs (Table 2 and Fig. 2).
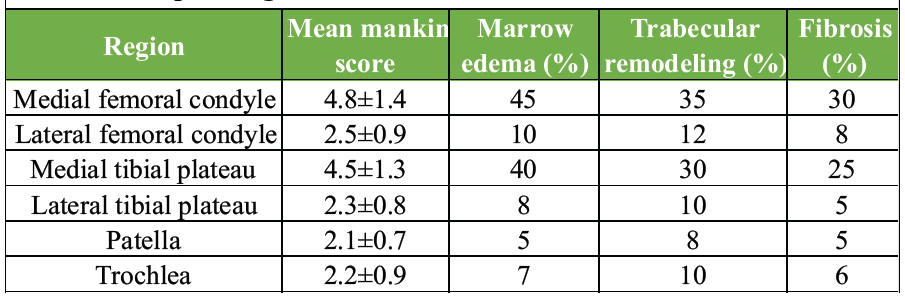
Table 2: Histopathological scores
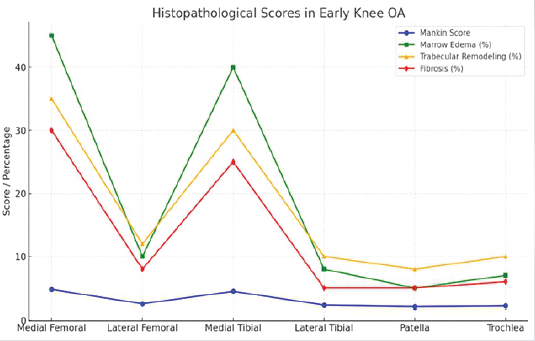
Figure 2: Histopathological scores.
MRI–histopathology correlation
Quantitative correlation analysis revealed a strong positive association between T2 relaxation times and Mankin scores in all cartilage regions (Pearson’s r = 0.72, P < 0.001). T1ρ relaxation times also showed a significant correlation with proteoglycan depletion (r = 0.69, P < 0.001). Semiquantitative MRI grading of BMLs demonstrated moderate agreement with histological marrow edema scores (Cohen’s kappa = 0.61). Correlation was strongest in the medial compartment, reflecting the typical pattern of early knee OA (Fig. 3).
Figure 3: Magnetic resonance imaging – histopathology correlation.
In the present study, MRI findings of cartilage and subchondral bone correlated well with histopathological changes in patients with early knee OA. Elevated T2 and T1ρ relaxation times were associated with higher Mankin scores, suggesting that quantitative MRI is sensitive to biochemical cartilage degeneration, such as proteoglycan loss and collagen disruption. These results are specific to early-stage OA, as the study exclusively included KL grade I–II patients. Therefore, the applicability of the findings to advanced OA cases remains limited.
Our results are in line with earlier studies. Li et al. reported that T1ρ mapping is more sensitive than T2 mapping for detecting early proteoglycan depletion in cartilage, showing strong correlation with histological assessment [12]. Regatte et al. also demonstrated significant correlation of quantitative MRI parameters with cartilage matrix changes in early OA [13]. Similar to our findings, Souza et al. observed that compositional MRI markers reflect early biochemical alterations even in the absence of major morphological defects [14]. The study evaluated only T2 and T1ρ quantitative mapping. Incorporating additional advanced MRI techniques, such as dGEMRIC, T2, or UTE imaging in future studies may provide a more comprehensive assessment of cartilage and bone composition.
With respect to subchondral bone, BMLs seen on MRI corresponded with marrow edema and remodeling on histopathology. Zanetti et al. showed that BMLs represent a spectrum of histological abnormalities, including necrosis, fibrosis, and trabecular changes [15]. Hunter et al. further confirmed that BMLs are associated with pain and predict structural progression in knee OA [16]. Our study supports these observations by demonstrating significant agreement between MRI-detected BMLs and histological marrow changes.
The strength of this study lies in the region-wise MRI–histology correlation, which enhances the biological validity of imaging biomarkers. However, the relatively small sample size and potential for spatial mismatch between MRI slices and biopsy specimens remain limitations. MRI demonstrated a strong correlation with histopathological features of early knee OA. Quantitative MRI markers, such as T2 and T1ρ can serve as reliable surrogates for biochemical cartilage degeneration, while MRI-detected BMLs reflect underlying histological changes. These findings highlight the role of MRI as a sensitive and non-invasive tool for early diagnosis and monitoring of OA.
Limitations of the study
- Small sample size (n = 40), limiting statistical power and generalizability.
- Cross-sectional design, preventing assessment of disease progression or predictive value of MRI biomarkers
- Potential spatial mismatch between MRI slices and biopsy sites
- Sampling bias due to tissue collection only from surgically accessible regions
- Possible histological artifacts from fixation, decalcification, and sectioning
- Inclusion restricted to early OA (KL I–II), limiting applicability to advanced disease
- Interobserver reproducibility for MRI and histopathology was not evaluated
- Limited MRI parameters assessed (only T2 and T1ρ mapping)
- Important confounding variables (BMI, alignment, activity, metabolic factors) were not controlled
- Single-time-point imaging and histology preventing assessment of temporal changes.
MRI showed a strong correlation with histopathological changes in early knee OA. Quantitative MRI parameters (T2 and T1ρ mapping) were significantly associated with proteoglycan loss and collagen disruption on histology, while MRI-detected BMLs corresponded with marrow edema and trabecular remodeling. These findings establish MRI as a sensitive, non-invasive tool for the early detection and monitoring of OA. Larger longitudinal studies are warranted to validate these results and explore the predictive role of MRI biomarkers in disease progression.
Quantitative MRI markers, such as T2 and T1ρ mapping can serve as reliable surrogates for early biochemical cartilage and subchondral bone changes, enabling earlier diagnosis, risk stratification, and monitoring of knee osteoarthritis progression – potentially guiding timely initiation of disease-modifying interventions.
References
- 1. Li X, Cheng J, Lin K, Saadat E, Bolbos RI, Jobke B, et al. Quantitative MRI using T1ρ and T2 in human osteoarthritic cartilage specimens: correlation with biochemical measurements and histology. Magn Reson Imaging. 2011;29(3):324–334 [Google Scholar] [PubMed]
- 2. Muratovic D, Findlay DM, Cicuttini FM, Wluka AE, Lee YR, Edwards S, et al. Bone marrow lesions in knee osteoarthritis: Regional differences in tibial subchondral bone microstructure and their association with cartilage degeneration. Osteoarthritis Cartilage 2019;27:1653-62. [Google Scholar] [PubMed]
- 3. MacKay JW, Kapoor G, Driban JB, Lo GH, McAlindon TE, Toms AP, et al. Association of subchondral bone texture on magnetic resonance imaging with radiographic knee osteoarthritis progression: data from the Osteoarthritis Initiative Bone Ancillary Study. Eur Radiol. 2018;28(11):4687–4695. [Google Scholar] [PubMed]
- 4. Li X, Cheng J, Lin K, Saadat E, Bolbos RI, Jobke B, et al. Quantitative MRI using T1ρ and T2 in human osteoarthritic cartilage specimens: correlation with biochemical measurements and histology. Magn Reson Imaging. 2011;29(3):324–334. [Google Scholar] [PubMed]
- 5. Binks DA, Hodgson RJ, Ries ME, Foster RJ, Smye SW, McGonagle D, et al. Quantitative parametric MRI of articular cartilage: a review of progress and open challenges. Br J Radiol. 2013;86(1023) [Google Scholar] [PubMed]
- 6. Joseph GB, Baum T, Alizai H, Carballido-Gamio J, Nardo L, Virayavanich W, et al. Baseline mean and heterogeneity of MR cartilage T2 are associated with morphologic degeneration of cartilage, meniscus, and bone marrow over 3 years–data from the osteoarthritis initiative. Osteoarthritis Cartilage 2012;20:727-35. [Google Scholar] [PubMed]
- 7. Guermazi A, Hayashi D, Roemer FW, Zhu Y, Niu J, Crema MD, et al. Synovitis in knee osteoarthritis assessed by contrast-enhanced magnetic resonance imaging (MRI) is associated with radiographic tibiofemoral osteoarthritis and MRI-detected widespread cartilage damage: the MOST study. J Rheumatol. 2014;41(3):501–508. [Google Scholar] [PubMed]
- 8. Albano D, Viglino U, Esposito F, Rizzo A, Messina C, Gitto S, et al. Quantitative and compositional MRI of the articular cartilage: a narrative review. Tomography. 2024;10(7):949–969. [Google Scholar] [PubMed]
- 9. Binks D, Wyman BT, Hudelmaier M. Quantitative parametric MRI of articular cartilage: A review of progress and open challenges. Osteoarthritis Cartilage 2013;21:711-21. [Google Scholar] [PubMed]
- 10. Stefanik JJ, Gross KD, Guermazi A, Felson DT, Roemer FW, Zhang Y, et al. The relation of MRI-detected structural damage in the medial and lateral patellofemoral joint to knee pain: the Multicenter and Framingham Osteoarthritis Studies. Osteoarthritis Cartilage. 2015;23(4):565–570. [Google Scholar] [PubMed]
- 11. Souza RB, Stehling C, Wyman BT, Hellio Le Graverand MP, Li X, Link TM, et al. The effects of acute loading on T1ρ and T2 relaxation times of tibiofemoral articular cartilage. Osteoarthritis Cartilage. 2010;18(12):1557–1563. [Google Scholar] [PubMed]
- 12. Li X, Benjamin Ma C, Link TM, Castillo DD, Blumenkrantz G, Lozano J, et al. In vivo T1ρ and T2 mapping of articular cartilage in osteoarthritis of the knee using 3T MRI. Magn Reson Med 2008;59:532-9. [Google Scholar] [PubMed]
- 13. Regatte RR, Akella SV, Lonner JH, Kneeland JB, Reddy R. T1rho relaxation mapping in human osteoarthritis (OA) cartilage: Comparison of T1rho with T2. J Magn Reson Imaging 2006;23:547-53. [Google Scholar] [PubMed]
- 14. Souza RB, Kumar D, Calixto N, Singh J, Schooler J, Subburaj K, et al. Response of knee cartilage T1rho and T2 relaxation times to in vivo mechanical loading in individuals with and without knee osteoarthritis. Osteoarthritis Cartilage 2012;20:1367-73. [Google Scholar] [PubMed]
- 15. Zanetti M, Bruder E, Romero J, Hodler J. Bone marrow edema pattern in osteoarthritic knees: Correlation between MR imaging and histologic findings. Radiology 2000;215:835-40. [Google Scholar] [PubMed]
- 16. Hunter DJ, Zhang Y, Niu J, Goggins J, Amin S, LaValley MP, et al. Increase in bone marrow lesions associated with cartilage loss: A longitudinal magnetic resonance imaging study of knee osteoarthritis. Arthritis Rheum 2006;54:1529-35. [Google Scholar] [PubMed]