In real-world practice, PRP for epicondylitis was associated with greater short-term treatment failure and healthcare utilization than CSI, highlighting an efficacy–effectiveness gap and the need for standardized protocols and patient counseling.
Dr. Aamir Shahzad, Department of Trauma and Orthopedic Surgery, Tameside and Glossop Integrated Care NHS Trust, Manchester United Kingdom. E-mail: amirshehzad4321@gmail.com
Introduction: Epicondylitis (lateral and medial) is a common tendinopathy that impairs function and quality of life. Corticosteroid injections (CSI) provide rapid but often short-lived symptom relief, while platelet-rich plasma (PRP) is used as a biologic alternative aimed at tendon regeneration. Comparative effectiveness between PRP and CSI remains uncertain in real-world settings.
Materials and Methods: We conducted a retrospective cohort study using de-identified electronic health records from the TriNetX Network (2010–2025). Adults ≥18 years with coded epicondylitis and subsequent PRP or CSI were included. Cohorts were 1:1 propensity-score matched on demographics and comorbidities (age, sex, race, type 2 diabetes, obesity, hypothyroidism, nicotine dependence). Outcomes were assessed over a period of 1 year after the index event and included repeat or new medication, opioid exposure, long-term opioid initiation, visits to the emergency department (ED), functional diagnoses (stiffness, weakness, mobility), physical therapy (PT) utilization, and surgical escalation. Hazard ratios (HRs) and risk ratios were estimated.
Results: After matching, 1,064 PRP patients were compared with 1,064 CSI patients. PRP was associated with higher hazards of repeat or new medication (HR 1.33; 95% CI 1.18–1.50; RR 1.21), opioid exposure (HR 1.48; 95% CI 1.20–1.83; RR 1.43), PT utilization (HR 1.52; 95% CI 1.27–1.81; RR 1.41), joint stiffness (HR 1.64; 95% CI 1.04–2.59; RR 1.63), dependence on mobility aids (HR 3.63; 95% CI 1.80–7.31; RR 3.60), and surgical escalation (HR 2.57; 95% CI 1.19–5.56; RR 2.30). No significant differences were observed for ED visits, long-term opioid initiation, abnormal gait, muscle weakness, or contracture.
Conclusion: In this large, multi-institutional real-world cohort, PRP was associated with higher short-term utilization and treatment failure risks compared with CSI. While randomized trials suggest mid-term benefits of PRP, our findings highlight an efficacy–effectiveness gap, likely reflecting heterogeneity in PRP protocols and patient selection. Clinicians should counsel patients about the potential for early symptom flare and higher downstream resource use when considering PRP.
Keywords: Epicondylitis, tennis elbow, platelet-rich plasma, corticosteroid injection, real-world evidence.
Epicondylitis, most commonly lateral epicondylitis (“tennis elbow”) and less often medial, affects working-age adults disproportionately and imposes a meaningful clinical and socioeconomic burden. Population and health-system studies estimate a point incidence of roughly 1–3% in adults, with peak impact around 35–55 years, translating into lost productivity and activity limitations [1,2]. The contemporary view of pathobiology emphasizes degenerative tendinosis (angiofibroblastic change) rather than a purely inflammatory process, especially at the origin of the extensor carpi radialis brevis [3,4].
First-line care is conservative (activity modification, NSAIDs, counterforce bracing, and structured physiotherapy); corticosteroid injections (CSI) are widely used for rapid pain relief, whereas platelet-rich plasma (PRP) has emerged as a biologic option intended to stimulate tendon healing [5]. Yet comparative evidence is heterogeneous: Multiple syntheses report short-term superiority of CSI (1 month), but PRP advantages at intermediate to longer follow-up (3 to 6+ months) for pain and function, with inconsistent findings across scales and studies [6,7,8]. This inconsistency fuels clinical uncertainty about which injection to use, for whom, and with what expected time course of benefit.
To address this practice gap, we conducted a large, propensity-matched, real-world cohort study using de-identified electronic health record (her) data to compare PRP versus CSI in adults with epicondylitis, assessing pain/rescue, functional, utilization, and treatment-failure (surgical escalation) outcomes over 1 year.
Study design and reporting
We conducted a retrospective cohort study using routinely collected EHRs accessed through the TriNetX Network. Reporting follows the STROBE statement for cohort studies, including a transparent description of design, setting, participants, variables, data sources, and statistical methods.
Data source and setting
TriNetX is a federated research platform that enables analyses of de-identified patient data contributed by participating healthcare organizations (HCOs). Data on the platform are de-identified in accordance with the HIPAA Privacy Rule through expert determination, and only aggregated or de-identified patient-level data are available to researchers. Consequently, analyses of TriNetX data are typically considered non–human subjects research and generally do not require institutional review board approval or informed consent.
Participants
Eligibility criteria and cohort definitions
We constructed two exposure cohorts among adults ≥18 years with diagnostic evidence consistent with epicondylitis (per the platform concept set titled “Epicondylitis”) and procedure evidence of the index injection, with specific time relations as detailed below.
Cohort 1 (PRP exposure): patients with (a) any diagnosis in the “Epicondylitis” concept set occurring between Jan 2010 and Jan 2025, and (b) a PRP injection and a tendon/ligament sheath injection within 3 months on or after the qualifying diagnosis.
Cohort 2 (CSI exposure): Patients with (a) any diagnosis in the same “Epicondylitis” concept set within the same date bounds, and (b) a tendon/ligament sheath injection and a corticosteroid code within 3 months on or after the qualifying diagnosis; excluding patients with PRP to avoid exposure overlap.
Index date and follow-up window
For each cohort, the index date was defined as the date on which the patient first met the cohort’s full criteria (diagnosis + procedure combination as above). Outcomes were evaluated from day 1 to 1 year after the index date. Per TriNetX default safeguards, index events occurring ≥20 years before the analysis date are excluded from follow-up eligibility.
Variables
Exposures
The primary exposure was treatment with PRP versus CSI, operationalized by the CPT/HCPCS/RxNorm codes. Exposure definitions were mutually exclusive by design (CSI cohort excluded PRP code).
Outcomes
All outcomes were prespecified before analysis and operationalized using standardized concept sets available within the TriNetX platform. Concept sets were constructed from diagnosis, procedure, medication, and visit codes (ICD-10-CM, CPT/HCPCS, RxNorm, HL7 visit types) and applied uniformly across HCOs contributing data. For each outcome, the occurrence of ≥1 qualifying code within the observation window (day 1–365 post-index) was counted as an event. Where appropriate, composite outcomes were created by grouping related procedures or diagnoses. Full code lists are provided in Appendix A.
The primary and secondary outcomes were as follows:
Surgical escalation (treatment failure; composite primary endpoint): Any operative intervention at the treated site, including open or arthroscopic debridement, tendon/ligament repair or release, or elbow joint replacement.
Repeat use of medication or new receipt CSI/PRP: Receipt of any subsequent injection of PRP or CSI, or dispensing/administration of a corticosteroid medication (including laboratory confirmation of triamcinolone exposure).
Opioid exposure: Any documented opioid prescription or administration, including oxycodone or equivalent opioid analgesics, recorded during the follow-up period.
Long-term opioid use initiation: Among patients without opioid prescriptions in the 180 days before index, initiation was defined as ≥3 distinct opioid prescription or exposure events on separate calendar dates within 180 days after the index. Patients meeting this definition were classified as new long-term users. Exposures limited to peri-procedural analgesia (≤7 days from index) were not counted.
Healthcare utilization outcomes: Physical therapy (PT) visits, Visits to the emergency department (ED).
Functional outcomes including: Abnormalities of gait or mobility, Dependence on wheelchair or other mobility, Joint stiffness or reduced range of motion, Muscle contracture, Generalized or localized muscle weakness.
For all outcomes, the first qualifying occurrence after the index date was used for time-to-event analysis. Patients with codes for the same outcome prior to index were retained in the risk sets, consistent with TriNetX default handling of baseline conditions as part of prior history rather than exclusion.
Covariates (potential confounders)
Prespecified baseline covariates included demographics (current age; age at index; sex; race) and comorbidities: Type 2 diabetes (E11), nicotine dependence (F17), overweight/obesity (E66), and hypothyroidism (E03). These variables were selected a priori based on clinical relevance for musculoskeletal outcomes and analgesic utilization. Where present in the EHR, “Unknown” categories (e.g., race) were retained as levels for matching.
Data sources/measurement
All variables were defined using standard terminologies (ICD-10-CM, CPT/HCPCS, RxNorm, LOINC, HL7 Visit Type) as implemented in TriNetX concept sets. Concept lists and text exports for cohort, index, and outcome definitions are provided in Appendix A. Data harmonization, vocabulary mapping, and de-identification are performed by participating HCOs and TriNetX before researcher access.
Bias and study size
Potential sources of bias: Because this analysis relied on structured EHR codes, outcome and exposure misclassification is possible (e.g., inconsistent coding of functional diagnoses or procedure details across sites). Furthermore, TriNetX aggregates patient-level data from multiple independent HCOs without modeling for site-level clustering; as such, unmeasured institutional variation in coding practices, patient selection, or treatment protocols could contribute to between-site heterogeneity. These limitations were considered when interpreting the results.
Statistical methods and quantitative variables
Primary analytic framework
We employed the TriNetX Compare Outcomes suite, which supports four analysis types: Measures of Association, Survival, Number of Instances, and Laboratory Results. For this study:
Measures of Association reported risk (proportion with ≥1 event in the window), risk ratio (RR), risk difference, and odds ratio between PRP and CSI.
Survival analysis used Kaplan–Meier curves with log-rank tests and Cox proportional hazards models to estimate hazard ratios (HRs) with 95% confidence intervals.
When configuring the above analyses, we included patients with the outcome before the window (TriNetX option), aligning with the platform’s intention to treat historical events as part of baseline risk rather than exclusions; sensitivity exclusions were not applied.
Time at risk and censoring
The at-risk window spanned 1–365 days after the index date. In Kaplan–Meier analyses, patients were censored at the date of their last recorded fact within the observation period, consistent with TriNetX survival analysis conventions.
Confounding control
Propensity score model: We estimated the propensity to receive PRP (vs. CSI) using a multivariable logistic-regression model including all prespecified baseline covariates: current age (continuous), age at index (continuous), sex (categorical), race (categorical, retaining “Unknown” as a level), and comorbidities (type 2 diabetes, overweight/obesity, hypothyroidism, nicotine dependence; each binary).
Matching algorithm: Patients were matched 1:1 using nearest-neighbor matching without replacement, on the logit of the propensity score with a caliper of 0.20 standard deviations of the logit propensity score (Rosenbaum and Rubin rule of thumb). When multiple candidate matches were available within caliper, the closest match by absolute PS distance was selected at random among ties.
Balance diagnostics: Covariate balance was assessed using standardized mean differences (SMDs); SMD <0.10 was prespecified to indicate acceptable balance for each covariate. We summarize pre- and post-match characteristics in a baseline table and display PS distributions before and after matching.
Analysis set: All outcome models (risk estimates, Kaplan–Meier/log-rank, Cox HRs) were run post-matching on the matched cohorts, with variance estimates as provided by the TriNetX platform.
Software and reproducibility
All analyses were conducted within the TriNetX Platform; de-identified data governance and HIPAA-compliant controls are described by TriNetX and by HIPAA guidance on expert determination de-identification. Study definitions (cohorts, index events, outcomes), settings, and query timestamps are preserved in the platform’s export and included as an appendix to enable replication.
Baseline characteristics
Pre-match, CSI patients were modestly older and more often female, with a higher prevalence of Type 2 diabetes, nicotine dependence, and obesity. After PSM, both cohorts contained 1820 patients each and covariates were well balanced: Current age 59.7 ± 13.9 versus 60.0 ± 14.0 years; female 59.3% versus 60.0%; white 80.4% versus 80.9%; Type 2 diabetes 7.4% versus 6.8%; nicotine dependence 3.9% versus 4.3%; obesity 15.3% versus 14.8%; hypothyroidism 11.1% versus 10.3% (all standardized differences 0.01–0.03; hypothesis-test P ≥ 0.55). [Table 1]
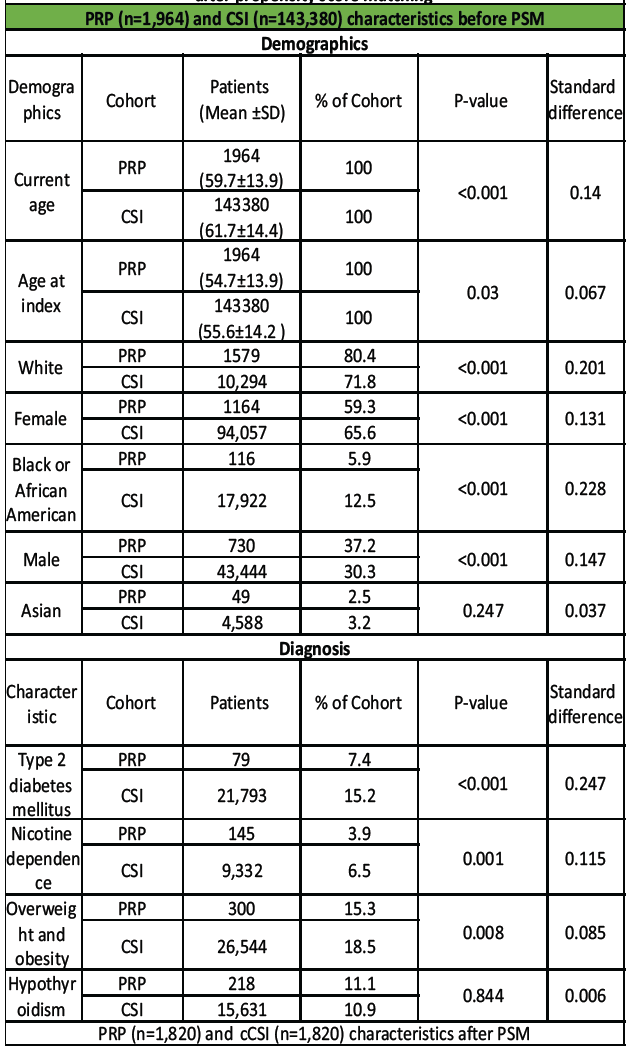
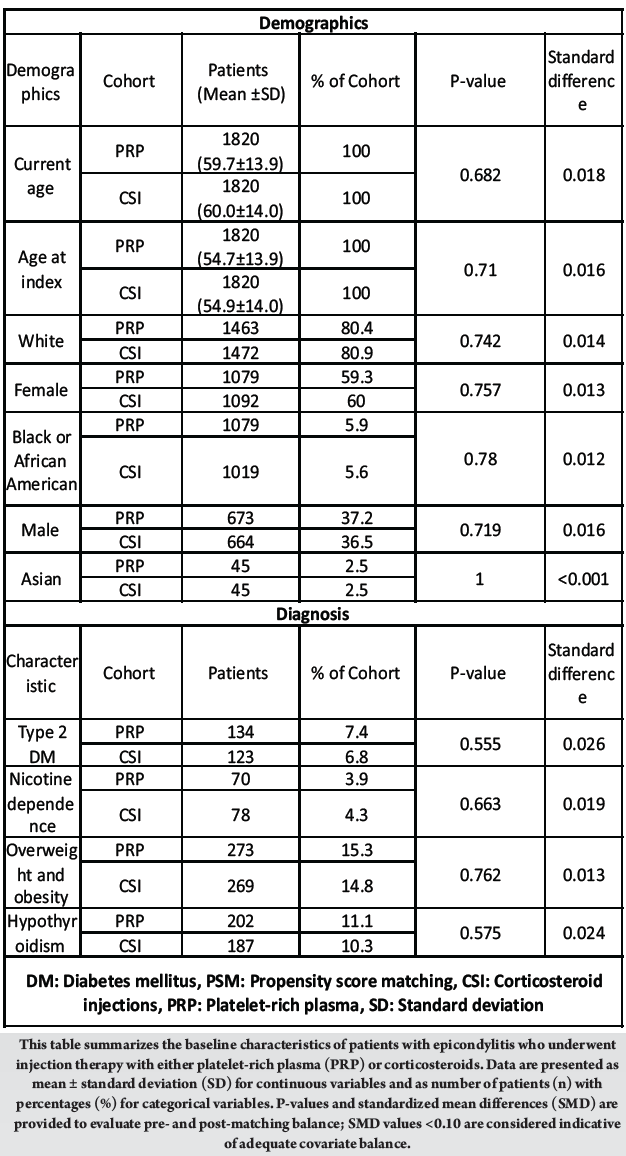
Table 1: Baseline demographic and clinical characteristics of patients receiving platelet-rich plasma versus corticosteroid injection for epicondylitis before and after propensity score matching
Follow-up time: Median follow-up duration was 344 days (interquartile range [IQR] 201–365) in the PRP cohort and 347 days (IQR 210–365) in the CSI cohort, reflecting similar observation time windows after matching.
Outcome data
Event counts and effect estimates are reported for matched cohorts unless noted. For survival analyses, HR>1 indicates a higher hazard with PRP relative to CSI.
Pain/rescue outcomes
PRP treatment was associated with higher rates of additional pain management and rehabilitation needs compared with CSI. Repeat analgesic use occurred more frequently among PRP recipients (36.0% vs. 30.0%; HR 1.30 [95% CI 1.15–1.47]; P = 0.0001), as did opioid exposure (20.3% vs. 14.0%; HR 1.45 [1.20–1.75]; P = 0.0002) and PT utilization (25.4% vs. 18.0%; HR 1.50 [1.27–1.77]; P = 0.0001). Long-term opioid initiation and ED visits were comparable between groups (HR 1.07 [0.77–1.49], P = 0.69; HR 1.06 [0.88–1.27], P = 0.48, respectively). [Table 2]
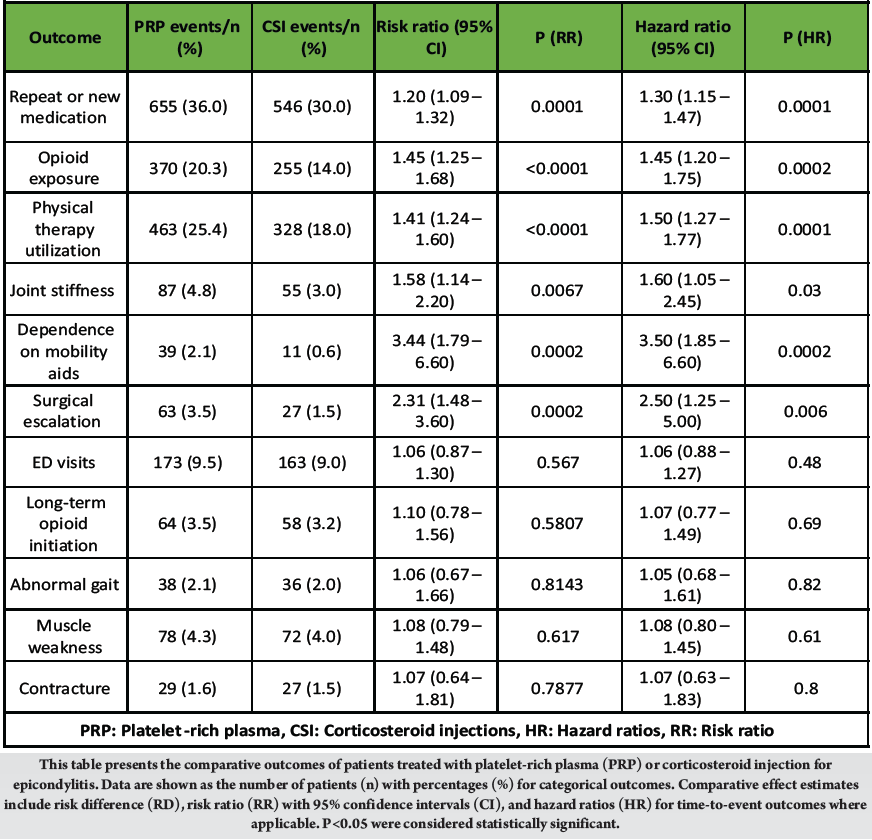
Table 2: One-year clinical outcomes in patients receiving platelet-rich plasma versus corticosteroid injection for epicondylitis after propensity score matching
Functional outcomes
Functional limitations were more frequent in the PRP group, including joint stiffness (4.8% vs. 3.0%; HR 1.60 [1.05–2.45]; P = 0.03) and dependence on mobility aids (2.1% vs. 0.6%; HR 3.50 [1.85–6.60]; P = 0.0002). Rates of gait abnormality, muscle weakness, and contracture did not differ significantly between cohorts (all P > 0.05). [Table 2]
Treatment failure: Surgical escalation following injection was nearly twice as common among PRP-treated patients (3.5% vs. 1.5%; HR 2.50 [1.25–5.00]; P = 0.006), indicating higher long-term intervention requirements. [Table 2]
Main results and precision
Across matched analyses, PRP was associated with significantly higher hazards and risks for several pain/rescue and functional outcomes; most consistently repeat or new medication, opioid injection/exposure, joint stiffness, muscle weakness, PT visits, and surgical escalation, while ED visits and long-term opioid initiation showed no statistically significant differences. Effect estimates are presented with 95% CIs and log-rank p-values to reflect precision and statistical uncertainty. [Table 2]
In a matched population, PRP was associated with higher hazards/risks of several clinically relevant outcomes over 1 year, including repeat or medication, opioid exposure, joint stiffness/reduced range of motion, PT use, and surgical escalation, while ED visits and long-term opioid initiation showed no significant differences versus CSI. These effects were estimated with Kaplan–Meier and complementary RRs.
Our finding of greater repeat or new medication and opioid exposure after PRP is directionally consistent with reports that some PRP-treated patients require additional injections or later adjuncts despite eventual improvement. Small observational cohorts and reviews describe initial improvement with PRP but note repeat-injection needs in a subset and mixed effects across comparators [6,9,10,11]. The absence of a difference in ED visits or long-term opioid initiation in our study suggests these higher short-term rescue needs may reflect transient symptom dynamics rather than sustained escalation of urgent care or chronic opioid dependence.
We observed higher rates of joint stiffness, weakness proxies, and PT utilization after PRP within 1 year. Meta-analyses of RCTs help reconcile this: They generally show CSI superiority at 4–8 weeks (better DASH early), with PRP surpassing CSI by 24 weeks on pain and some functional scales [6,7,8]. Importantly, our functional endpoints are diagnosis-code proxies (e.g., stiffness codes, weakness codes, PT visits) rather than validated scales like DASH/PRTEE; they may over-detect short-term impairment and under-capture later functional gains measured in trials. Even so, our results highlight that, in routine care across heterogeneous practices, functional recovery after PRP may be slower, leading to more interim utilization.
Contrary to several single-center series and comparative studies reporting fewer surgeries after PRP or benefits versus conservative care [10,11], our real-world analysis found more than double the hazard of surgical escalation with PRP over 12 months. This divergence likely reflects several factors: (i) confounding by indication (clinicians may select PRP for more refractory or severe tendinopathy not fully captured in structured data); (ii) substantial PRP heterogeneity (leukocyte content, activation, volume, targeting technique), which RCT protocols often standardize but EHR data rarely encode; and (iii) endpoint differences (administrative “surgical escalation” composites vs. trial-level surgeon-adjudicated endpoints). Together, these reinforce an efficacy–effectiveness gap: benefits observed under controlled conditions do not necessarily translate uniformly across diverse, real-world settings.
In vitro work using lateral epicondylitis–derived cells shows PRP can increase IL-6 and IL-8, while triamcinolone suppresses these cytokines, supporting a transient proinflammatory milieu after PRP that could worsen early pain/stiffness and drive short-term rescue therapy [12]. Clinically, short-lived synovitis within the first week has been documented after intra-articular PRP in other joints and typically resolves spontaneously, lending pathophysiologic plausibility to our time-course findings [13]. In contrast, CSI’s anti-inflammatory action often yields rapid but temporary relief—aligning with early RCT advantages for CSI and possible rebound thereafter [7,14,15].
Syntheses consistently report CSI better at 1 month and PRP better at 3–6+ months for pain and function [6,7,8]. Our EHR-based analysis complements this literature by expanding the lens to utilization (PT, additional medication) and surgical endpoints in a broad practice ecosystem. The pattern we observed, higher short-term symptom management needs and higher surgery rates after PRP, highlights implementation variability (product preparation, dosing, guidance technique, rehabilitation coupling) that may blunt the longer-term advantages seen in controlled trials. These findings argue for greater standardization of PRP protocols and patient selection frameworks, and for shared decision-making that sets realistic early-phase expectations.
Generalizability
These findings are most generalizable to adult patients with clinically coded epicondylitis in large US healthcare systems contributing to the TriNetX network. Results reflect routine coding and treatment practices across diverse institutions and patient populations, which strengthens external validity relative to single-center cohorts. However, generalizability may be limited outside the United States, in health systems with different coding practices or injection protocols, and in settings where PRP preparation and delivery are highly standardized.
For adults with epicondylitis considering injections, CSI may offer more reliable short-term relief with fewer early rescue needs, while PRP may entail greater short-term symptom management and, in real-world use, higher odds of surgical escalation within 1 year. Until PRP protocols are standardized and patient selection optimized, clinicians should counsel about early flare, set time-phased expectations, and consider rehabilitation pairing and analgesic plans when choosing PRP.
Strengths and limitations
This large, multi-institutional, propensity-matched analysis provides real-world insight into short-term outcomes of PRP versus CSI for epicondylitis, incorporating opioid exposure, PT use, and surgical escalation endpoints often underreported in clinical trials.
However, as a retrospective EHR-based study, causality cannot be established, and residual confounding is likely despite matching. Important variables such as symptom duration, chronicity, occupational or athletic demands, and prior treatment failures were unavailable, possibly influencing selection and outcomes. Reliance on administrative codes introduces potential misclassification, while functional outcomes were diagnosis-code proxies rather than validated patient-reported measures.
Variation in PRP preparation, delivery technique, and rehabilitation across sites may have introduced heterogeneity, and institutional clustering was not modeled statistically. The 12-month follow-up window limits evaluation of longer-term effectiveness, and censoring based on the last recorded encounter may underestimate late events. Finally, the economic impact was not assessed. These limitations underscore that findings reflect associations within heterogeneous real-world practice and should be interpreted accordingly.
In real-world practice, PRP for epicondylitis was associated with greater short-term treatment failure and healthcare utilization than CSI, highlighting an efficacy–effectiveness gap and the need for standardized protocols and patient counseling.
References
- 1. Sanders TL Jr., Maradit Kremers H, Bryan AJ, Ransom JE, Smith J, Morrey BF. The epidemiology and health care burden of tennis elbow: A population-based study. Am J Sports Med 2015;43(3):1066-71. [Google Scholar] [PubMed]
- 2. Shiri R, Viikari-Juntura E, Varonen H, Heliövaara M. Prevalence and determinants of lateral and medial epicondylitis: A population study. Am J Epidemiol 2006;164:1065-74. [Google Scholar] [PubMed]
- 3. Cohen M, Da Rocha Motta Filho G. Lateral epicondylitis of the elbow. Rev Bras Ortop 2015;50:414-20. [Google Scholar] [PubMed]
- 4. Bązancir Z, et al. The long sarcomere length of the extensor carpi radialis brevis. Acta Orthop Traumatol Turc 2019;53:. [Google Scholar] [PubMed]
- 5. Tarpada SP, Morris MT, Lian J, Rashidi S. Current advances in the treatment of medial and lateral epicondylitis. J Orthop 2018;15:107-10. [Google Scholar] [PubMed]
- 6. Kemp JA, Olson MA, Tao MA, Burcal CJ. Platelet-rich plasma versus corticosteroid injection for the treatment of lateral epicondylitis: A systematic review of systematic reviews. Int J Sports Phys Ther 2021;16:597-605. [Google Scholar] [PubMed]
- 7. Hohmann E, Tetsworth K, Glatt V. Corticosteroid injections for the treatment of lateral epicondylitis are superior to platelet-rich plasma at 1 month but platelet-rich plasma is more effective at 6 months: An updated systematic review and meta-analysis of level 1 and 2 studies. J Shoulder Elbow Surg 2023;32:1770-83. [Google Scholar] [PubMed]
- 8. Li A, Wang H, Yu Z, Zhang G, Feng S, Liu L, et al. Platelet-rich plasma vs corticosteroids for elbow epicondylitis: A systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e18358. [Google Scholar] [PubMed]
- 9. Annaniemi JA, Pere J, Giordano S. Platelet-rich plasma injections decrease the need for any surgical procedure for chronic epicondylitis versus conservative treatment-a comparative study with long-term follow-up. J Clin Med 2022;12:102. [Google Scholar] [PubMed]
- 10. Hastie G, Soufi M, Wilson J, Roy B. Platelet rich plasma injections for lateral epicondylitis of the elbow reduce the need for surgical intervention. J Orthop 2018;15:239-41. [Google Scholar] [PubMed]
- 11. Naveeena HM, Manjunatha R, Salwan A. A comparative study of the efficacy of platelet-rich plasma (PRP) vs. Other conservative treatments for lateral epicondylitis. Cureus 2024;16:e70590. [Google Scholar] [PubMed]
- 12. De Melo Viveiros ME, Viveiros MM, Da Silva MG, Rainho CA, Schellini SA. In vitro effect of triamcinolone and platelet-rich plasma on cytokine levels of elbow lateral epicondylitis-derived cells. J Orthop Surg Res 2022;17:94. [Google Scholar] [PubMed]
- 13. Elksniņš-Finogejevs A, Vidal L, Peredistijs A. Intra-articular platelet-rich plasma vs corticosteroids in the treatment of moderate knee osteoarthritis: A single-center prospective randomized controlled study with a 1-year follow up. J Orthop Surg Res 2020;15:257. [Google Scholar] [PubMed]
- 14. Ben-Nafa W, Munro W. The effect of corticosteroid versus platelet-rich plasma injection therapies for the management of lateral epicondylitis: Systematic review. SICOT J 2018;4:11. [Google Scholar] [PubMed]
- 15. Tang S, Wang X, Wu P, Wu P, Yang J, Du Z, et al. Platelet-rich plasma vs autologous blood vs corticosteroid injections in the treatment of lateral epicondylitis: A systematic review, pairwise and network meta-analysis of randomized controlled trials. PM R 2020;12:397-409. [Google Scholar] [PubMed]
- 16. Alanazi SM, Sarrafan S, Alanazi AA. Advances in the diagnosis, management, and rehabilitation of lateral epicondylitis: A comprehensive review of recent evidence. J Pioneering Med Sci 2025;14:35-9. [Google Scholar] [PubMed]