In cases of hip effusion in infants, it is crucial to broaden the differential diagnosis beyond common inflammatory conditions to include rare hematological disorders. This approach ensures accurate diagnosis and prevents unnecessary invasive interventions.
Dr. Elif Reyyan Cadircibasi, Department of Orthopaedics and Traumotology, Health Sciences University, Umraniye Training and Research Hospital, Istanbul - 34764, Turkey. E-mail: drelifreyyan@gmail.com
Introduction: Non-traumatic hip pain in pediatric patients necessitates urgent evaluation due to a wide differential diagnosis, as misdiagnosis can lead to severe complications. This case report details the first known documentation in literature where intra-articular hip bleeding was the initial presentation of hemophilia in an infant, emphasizing the importance of recognizing hematological disorders amid typical orthopedic presentations.
Case Report: A 6-month-old male infant with no significant medical history presented with unexplained pain and irritability. Clinical examination demonstrated restricted motion in the right hip, and imaging studies revealed hip effusion. Initial laboratory findings indicated a negative C-reactive protein; however, the activated partial thromboplastin time was prolonged, raising suspicion for a bleeding diathesis. Joint aspiration yielded hemorrhagic fluid, and subsequent tests confirmed a critically low level of coagulation factor VIII, leading to a diagnosis of hemophilia. The patient underwent treatment with factor VIII and was managed with a Pavlik harness, successfully restoring hip stabilization and range of motion.
Conclusion: This case highlights the critical need for healthcare providers to consider hemophilia in the differential diagnosis for infants presenting with hip effusion and abnormalities in hemostasis parameters. By documenting a rare case where hemophilia mimicked infection or inflammation, this report aims to improve awareness and diagnostic protocols in pediatric orthopedics. The findings advocate for a cautious approach to invasive procedures in infants when a bleeding disorder has not been ruled out, ultimately enhancing clinical outcomes and advancing our understanding of disease etiology in similar presentations.
Keywords: Hemophilia, septic arthritis, hip effusion, pediatric orthopedics, differential diagnosis
Non-traumatic hip pain is a common cause for urgent orthopedic evaluation in pediatric patients, requiring a comprehensive differential diagnosis that includes transient synovitis, septic arthritis, Legg-Calvé-Perthes disease, slipped capital femoral epiphysis, juvenile idiopathic arthritis, and tumors. The presence of hip joint effusion, in addition to pain, necessitates early diagnosis to determine the cause and urgently rule out septic arthritis to prevent joint damage. While the majority of children presenting with hip effusions are typically diagnosed with transient synovitis (over 85%), which often resolves spontaneously, up to 10% may be identified as having septic arthritis [1]. Key indicators for septic arthritis include severe hip pain, fever, elevated white blood cell count, and increased levels of C-reactive protein (CRP). However, it is crucial to note that up to 30% of children with septic arthritis may present with partial signs and symptoms, leading to overlapping physical examination findings, clinical signs, imaging results, and laboratory profiles with those of transient synovitis [2]. However, rare diseases may occasionally contribute to joint effusion and hip pathologies in infants and should be considered in select cases. Hemophilia A and B are rare X-linked inherited bleeding disorders caused by deficiencies of coagulation factors VIII and IX, respectively [3,4]. These disorders typically present early in life with bleeding events. During the newborn period, the most common site of bleeding is the circumcision. As the child grows, hemarthrosis affecting larger joints becomes more common. Intra-articular bleeding patterns vary with age, with the ankle most affected from infancy to 5 years, followed by the knees and elbows [5]. During the first 2 years of life, approximately 28.3% of children with hemophilia experience intra-articular bleeds, though hip involvement is uncommon [6]. This can lead to diagnostic challenges when evaluating hip effusion, particularly as other causes, such as transient synovitis or septic arthritis are more frequently considered. In this case report, we present a 6-month-old infant referred with pseudoparalysis of the hip resembling septic arthritis.
A 6-month-old, otherwise healthy boy was admitted to the pediatrics clinic of an external hospital with unexplained pain and irritability. During the physical examination, the patient demonstrated signs of agitation and distress, particularly with movement of the right hip, which exhibited a restricted range of motion. Notably, no signs of hyperemia or warmth were present upon palpation of the right hip, and the remainder of the physical examination was unremarkable. The parents reported that the infant’s restlessness began 1 day before the visit. Medical history revealed no significant findings. A full-body magnetic resonance imaging (MRI) had performed to identify the potential source of the pain, revealing an effusion in the right hip (Fig. 1c, d, e). The patient was referred to a Level I training and research hospital with a provisional diagnosis of septic arthritis.
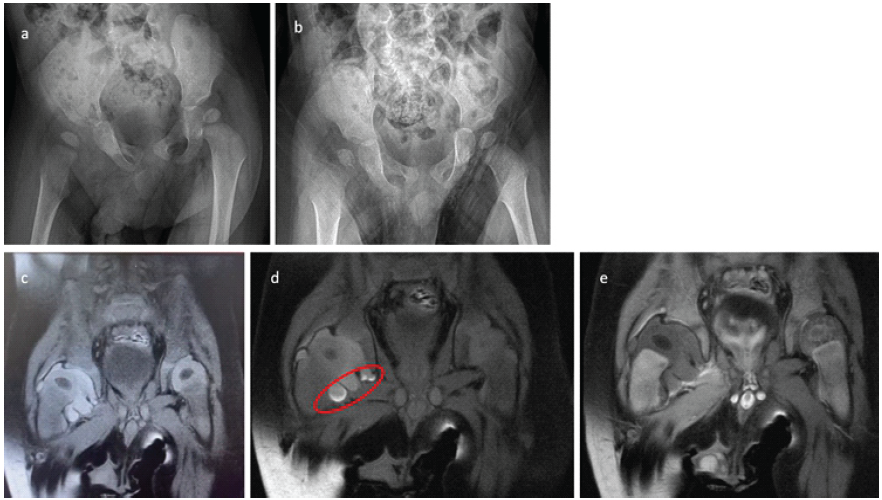
Figure 1: (a) Pre-operative X-ray showing subluxation of the right hip due to effusion. (b) Anteroposterior X-ray taken 12 h post-operatively showing relative relaxation of the right hip compared to the previous day. (c) T1-weighted, coronal fat-saturated magnetic resonance imaging (MRI) revealing a hyperintense effusion in the right hip. (d) T1-weighted, coronal fat-saturated MRI showing blood elements settled in the inferior part of the joint capsule (red circle). (e) T2-weighted, coronal fat-saturated MRI showing hypointense effusion in the right hip, indicative of a blood signal. Both femoral head ossification centers and epiphyses display normal signals, ruling out potential avascular necrosis.
Initial laboratory investigations at the external hospital indicated a negative CRP, a hemoglobin level of 10.5 g/dL, a hematocrit of 31.8%, a white blood cell count of 14,000 cells/µL, and a platelet count of 243,000 cells/µL. An X-ray of the pelvis demonstrated lateralization of the right femoral head (Fig. 1a and b), prompting further investigation with a hip ultrasound, which revealed effusion in the right hip. To clarify any infectious etiology, a decision was made to perform a joint aspiration under fluoroscopy. Before the aspiration, a blood sample was obtained for anesthesia preparation, resulting in an international normalized ratio (INR) of 1.23 and a prolonged activated partial thromboplastin time (aPTT) of 82 s. It is noteworthy that this aPTT value might have been overlooked before the aspiration. The patient underwent a decompression procedure through needle aspiration at the 3rd h following admission to the Level I hospital. During the fluoroscopy-guided aspiration, the aspirate was assessed to be hemorrhagic, and no purulent material was observed (Fig. 2).
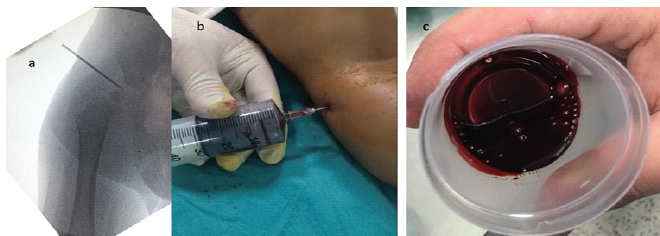
Figure 2: (a) Intraoperative fluoroscopy image showing the introduction of the needle into the right hip. (b) Intraoperative image demonstrating hip joint aspiration with the extraction of bloody effusion. (c) Collected 5 mL of bloody effusion from the right hip, indicative of possible hemarthrosis.
Concomitant microscopic examination revealed the presence of erythrocytes, a few leukocytes, and no detectable microorganisms. Following the exclusion of septic arthritis, joint debridement surgery was deemed unnecessary. The control X-ray in the next morning, revealed a more centralized femoral head. However, following the aspiration, there was a significant and sustained decline in hemoglobin levels. Consequently, the patient was referred to our Level I hospital for further evaluation and intervention, where specialized hematology and pediatric orthopedic services are available to address the situation. Upon arrival at our pediatric department, the patient was administered erythrocyte suspension and fresh frozen plasma. The prolonged aPTT, together with MRI findings were suggested to be indicative of hemarthrosis and the hemorrhagic nature of the aspirated joint fluid, raised strong suspicion for a hematological disorder, particularly hemophilia. Additional laboratory tests were subsequently conducted. During this period, the patient was evaluated in our department, where a Pavlik harness was applied for joint stabilization. Laboratory results revealed von Willebrand factor (VWF) at 98%, factor IX at 63%, and a markedly low factor VIII level of 4.4% (normal range: 70–150%), thereby confirming the diagnosis of hemophilia. In light of the joint bleeding episode, primary prophylaxis was initiated with 250 units of concentrated plasma-derived factor VIII. The pediatric infectious disease specialist recommended a continuation of antibiotic therapy for 20 days with vancomycin and ciprofloxacin until a definitive diagnosis was established to rule out septic arthritis. On the 20th day after aspiration, a follow-up MRI was performed, revealing that the femoral head had centralized with no effusion present (Fig. 3a and b).
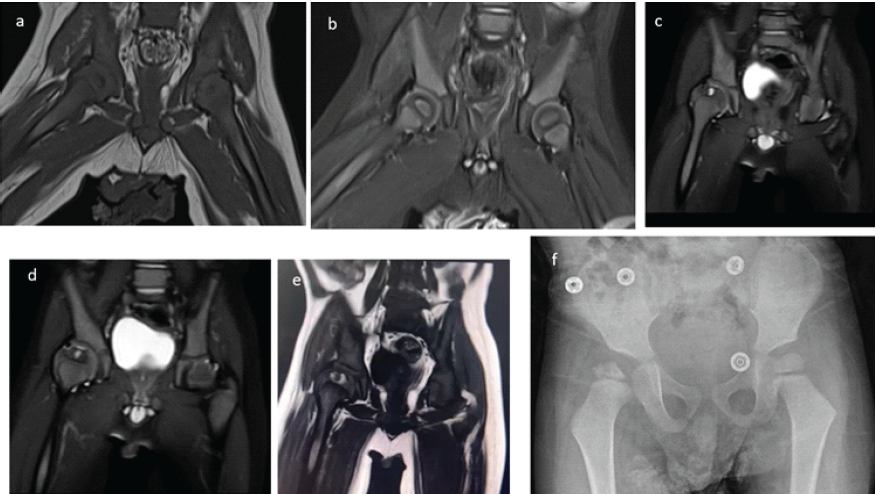
Figure 3: (a) T1-weighted coronal magnetic resonance imaging (MRI) showing the hip reduced to its normal position at post-operative 3 weeks. (b) Coronal short tau inversion recovery MRI showing both femoral heads in place without any signs of avascular necrosis. (c and e) T2-weighted coronal MRI obtained at post-operative 3 years, showing a cystic lesion with a surrounding hyperintense signal on the T1-weighted coronal image. These findings are indicative of hemophilic arthropathy (subchondral cyst caused by recurrent intra-articular hemorrhages leading to hemophilic arthropathy). (f) X-ray at post-operative 3 years showing the extent of deformity.
The patient was treated and followed for a total of 17 days in the hospital. Follow-up hematologic testing demonstrated negative Factor VIII inhibitor assays on two separate occasions, together with normal levels of VWF and Factor IX, fully supporting the diagnosis of Hemophilia A without inhibitor development. Following confirmation of hemophilia and satisfactory blood test results, he was discharged with detailed recommendations for the use of the Pavlik harness and instructions for follow-up at the outpatient clinic, along with a prescription to continue the 250 units of factor VIII. The Pavlik harness was utilized for a total duration of 12 weeks, during which the patient’s right hip demonstrated a full range of motion and no pain. A control ultrasound at the end of treatment showed a normal appearance of the hip joint. The patient continued to be followed up at 6-month intervals for potential femoral head avascular necrosis (AVN) or acetabular dysplasia. Throughout this follow-up period, the patient’s records did not document any significant spontaneous joint bleeding episodes; however, he did experience occasional transient joint pain episodes, which were successfully managed with factor VIII treatment. Three years post-operatively, a follow-up X-ray revealed irregularity proximal femoral epiphysis, characterized by a narrowed lateral pillar that retained over 50% of its height. The acetabular roof appeared normal, as indicated by an acceptable acetabular index (AI); however, irregularities were observed on the acetabular joint surface (Fig. 3f). A follow-up MRI, utilizing T2-weighted coronal images obtained at 3 years post-operation, demonstrated a cystic lesion accompanied by a surrounding hyperintense signal on the T1-weighted coronal images. These findings are suggestive of hemophilic arthropathy, specifically a subchondral cyst formed due to recurrent intra-articular hemorrhages (Fig. 3c, d, e). The X-ray taken in the 4th post-operative year illustrated concentric reduction of the femur along with flattening and remodeling of the femoral head. Furthermore, a slight steepening of the acetabulum was observed relative to both the contralateral hip and the imaging from the previous year. These findings are consistent with Catterall Grade 3 or Salter-Thompson Type 2b AVN (Fig. 4).
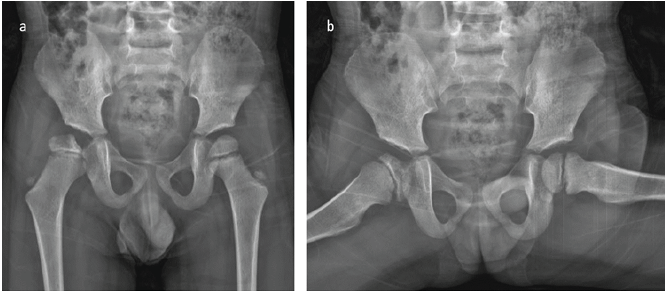
Figure 4: (a and b) X-ray at the 4th post-operative year showing concentric reduction of the femur with flattening and remodeling of the femoral head. Note the slight steepening of the acetabulum compared to the contralateral hip and the previous year. The findings resemble Catterall Grade 3 or Salter-Thompson Type 2b avascular necrosis.
All post-operative radiographs and MRI studies were reviewed to assess acetabular development, femoral head containment, epiphyseal height, and synovial thickness. To minimize observer bias, all quantitative radiologic measurements were independently performed by two authors, and mean values were used for analysis. Furthermore, all MRI examinations were evaluated and confirmed by a musculoskeletal radiology specialist with expertise in pediatric hip imaging. Radiographic evaluation at the 5th post-operative year, including contrast-enhanced MRI and X-rays, revealed regression of the previously observed subchondral cyst in the epiphysis (Fig. 5a,b). The perfusion of the epiphysis remained comparable to the healthy contralateral hip, similar to previous years, and the avascular area did not show any progression on MRI (Fig. 5a, b, c, d). The lateralization of the femoral head, attributed to synovial thickening, remained unchanged without further increase; however, no improvement in synovial thickening was noted either. Compared with the early post-operative X-rays from the 1st year (Fig. 1f), the femoral head demonstrated improved medialization at the 5th post-operative year, evidenced by increased metaphyseal overlap (Fig. 5e and f). The acetabular steepening observed at the 4th post-operative year was no longer present, suggesting a favorable correction in acetabular development.
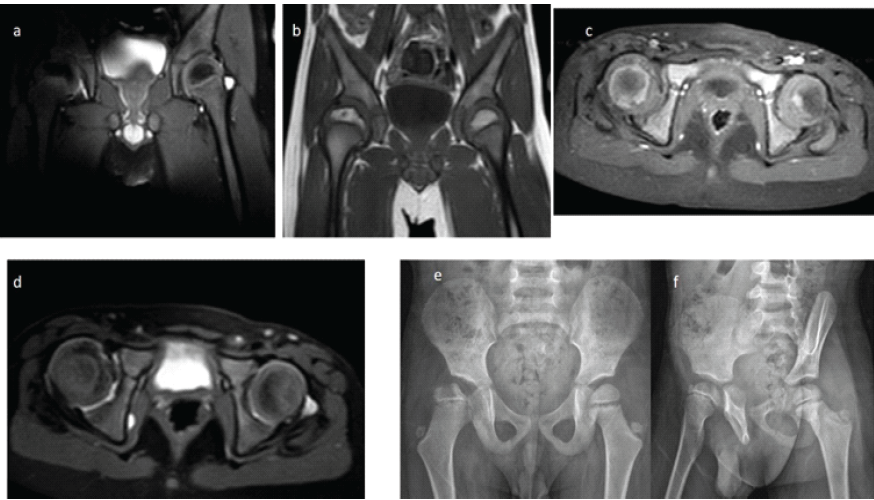
Figure 5: Radiographic evaluation of the patient at the 5th post-operative year. (a) T2-weighted coronal magnetic resonance (MR) image demonstrating similar signal intensity of the epiphysis, indicating limited necrosis of the right femoral head. (b) T1-weighted coronal MR image showing regression of the previously observed subchondral cyst. (c) Contrast-enhanced axial MR image revealing comparable enhancement patterns, suggestive of similar perfusion in both femoral heads. (d) Short tau inversion recovery axial MR image showing increased synovial tissue thickness on the right side without alteration in acetabular anteversion, indicating preserved acetabular development. (e) Anteroposterior pelvic radiograph demonstrating stable femoral head morphology compared with the previous year, with no increase in lateralization or evidence of acetabular dysplasia. (f) False-profile radiograph showing excellent anterior coverage of the femoral head.
The epiphyseal height ratio (injured/non-injured side), measured on coronal short tau inversion recovery MRI, was 0.86 at post-operative 3 months and 0.89 at post-operative 5 years. These findings indicate that the metaphyseal collapse did not worsen over time, although no substantial improvement was observed either. The medial synovial thickness ratio (injured/non-injured side), measured on coronal images, decreased from 1.53 at post-operative 3 months to 1.17 at post-operative 5 years, demonstrating a clear improvement in medial synovial thickening at the latest follow-up. The Reimer’s migration index, reflecting femoral head containment, was 44.7% at post-operative 3 years and improved to 48.8% and 52.1% at the 4- and 5-year follow-up radiographs, respectively. These values suggest that despite ongoing insufficiency, femoral head coverage gradually improved over time. The AI, used to assess acetabular development, measured 26° bilaterally at post-operative 6 months. At the post-operative 4th year, a developmental delay was noted in the right hip: While the AI of the left hip had improved to 23°, the right hip remained at 26°. By the post-operative 5th year, however, acetabular development in the right hip had accelerated, and both hips demonstrated a normalized AI of 21°, indicating recovery of acetabular growth on the affected side.
During long-term follow-up by the pediatric hematology team, serial laboratory tests confirmed stable hematological parameters, including normal hemoglobin (13.7 g/dL), platelet count (204 × 109/L), INR (1.04), and APTT (19.8 s), as well as Factor VIII levels maintained within therapeutic range under routine prophylaxis (77–95%), indicating effective control of bleeding risk throughout follow-up. During the 5th post-operative year, the patient was pain-free with full hip joint range of motion, no limb shortening, and the ability to walk and run without pain. However, the patient occasionally experiences a temporary limp when transitioning from sitting to standing, which resolves after walking a short distance. This issue is likely due to recurrent synovitis, potentially caused by hemarthrosis.
This case represents the first instance in the literature where intra-articular hip bleeding was the initial manifestation of hemophilia. Typically, hemophilia is diagnosed through one of three clinical scenarios: A positive family history, abnormal bleeding in the individual or close family members, or unexpected findings in coagulation tests [7]. In our case, there was no family history of hemophilia, which further delayed the diagnosis due to the rarity of hip involvement as an initial presentation and a lack of suspicion for a bleeding disorder. Although the acute phase reactants were negative, the presence of hip effusion initially led to the presumption of an inflammatory condition, including transient synovitis or septic arthritis. This reflects the diagnostic challenge, as hip involvement in hemophilia is exceedingly rare, whereas intra-articular bleeds usually affect the ankles, knees, and elbows [5]. The planned joint aspiration, however, resulted in a dramatic, life-threatening drop in hemoglobin levels. This highlights a critical consideration: Invasive procedures, such as joint aspiration, should be performed with caution in infants with unexplained effusion or pseudoparalysis, especially when bleeding diathesis has not been ruled out. It must also be acknowledged that the delayed recognition of the coagulation disorder in this case may have contributed to the clinical progression and the femoral head changes observed during follow-up. Earlier identification of hemophilia could potentially have altered the disease course, underscoring the importance of timely coagulation screening in infants presenting with atypical hip effusion. Retrospective evaluation revealed the presence of a bleeding diathesis, indicated by prolonged aPTT. Unfortunately, this key laboratory finding was initially overlooked, delaying the diagnosis of hemophilia. Neonates and infants with hemophilia are at an increased risk of bleeding events, including cerebral hemorrhage and joint or muscle hematomas, which may occur as early as the crawling or walking stage [8]. Bleeding disorders, although rare in hip effusions, must be included in the differential diagnosis, particularly in infants presenting with effusion-associated symptoms, such as pseudoparalysis. The pathophysiology of hemophilic arthropathy is not fully understood, particularly concerning early inflammation. The incidence of intra-articular bleeding varies based on the consistency of factor therapy, ranging from <1% to 7%. Repeated hemarthrosis leads to joint remodeling and cartilage damage, potentially resulting in bone cysts and instability [9,10]. Even a single episode of hemarthrosis can initiate an inflammatory process that results in synovial thickening and irreversible angiogenesis, increasing the risk of recurrent bleeding [11]. Floman and Niska [12] reported a case of a left hip dislocation following minor trauma in a 6-year-old boy with known hemophilia. The hip was reduced after factor VIII replacement therapy; nevertheless, the authors noted that the patient subsequently developed hip stiffness and ankylosis despite the reduction. In severe cases, bone resorption of the femoral head can occur due to disrupted blood flow in epiphyseal vessels from increased intracapsular pressure, which may lead to osteonecrosis instead of synovitis. The radiographic changes resemble those of Legg-Calvé-Perthes disease but have a poorer prognosis for revascularization in hemophilia, often resulting in secondary degenerative osteoarthritis [10,13]. Wallny et al. [14] in their study evaluating hemophilic children with avascular femoral head necrosis, followed seven patients for 5–50 years and found that most developed persistent clinical and radiographic abnormalities, including pain, limited motion, deformity, and subluxation. Although radiographic risk signs suggested a higher likelihood of later arthrosis, the small sample size limited definitive prognostic conclusions. The authors emphasized distinguishing hemophilic arthropathy from Legg-Calvé-Perthes disease, especially when acetabular changes are present. In our case, the features of femoral head AVN were obscure. The pattern of necrosis did not align with either Perthes disease or AVN following developmental dysplasia of the hip treatment. Interestingly, it more closely resembled the adult form of osteoarthritis, despite the patient still being a child. Moreover, the steepening of the acetabulum observed after 3 years of follow-up suggests that the acetabulum may also be involved. This occurred despite appropriate measures being taken to prevent acetabular dysplasia[15]. Nevertheless, at the post-operative 5th year, acetabular development was within normal limits, the previously observed central cystic lesion had regressed, and femoral head containment continued to improve. These favorable findings may be attributed to the early onset of intervention, allowing better remodeling potential in the immature hip. In addition, most previously published cases date back 3–5 decades, when hemophilia management strategies were less advanced; therefore, present factor replacement protocols may more effectively reduce recurrent intra-articular hemorrhagic episodes and secondary joint damage. However, it is important to emphasize that the patient is still very young, and substantial time remains until completion of skeletal maturity. Thus, long-term surveillance is essential before definitive conclusions can be made regarding the ultimate prognosis. Thus, it must be kept in mind that early-stage patients with intra-articular hip bleeding and hemophilia should be closely monitored, as intervention may be necessary to prevent further pathological changes. The late identification of intra-articular bleeding episodes in infants can lead to significant acute pain, functional limitations, and long-term consequences affecting mobility and overall quality of life. However, with early diagnosis and appropriate treatment, such as the administration of plasma-derived factor VIII and the use of a Pavlik harness for joint stabilization, patients can regain full hip joint motion, as demonstrated in this case. In retrospect, the empiric antibiotic therapy administered during the early phase of treatment was unnecessary, as no signs of infection were ever demonstrated; the patient’s improvement can instead be clearly attributed to factor VIII replacement correcting the previously unrecognized bleeding diathesis. This highlights the importance of a multifaceted approach in managing hemophilia in infants, including preventative therapy, vigilant monitoring, and timely interventions to address potential complications. Moreover, these patients should be followed closely until skeletal maturity, and families should be informed about the possibility of deformities that may arise.
Hemophilia should be included in the differential diagnosis of infants presenting with symptoms resembling septic arthritis or transient synovitis of the hip, particularly when abnormalities in hemostasis parameters are present. Although pre-operative evaluations may not always provide a definitive diagnosis, careful attention to subtle findings can lead to timely and accurate identification, ensuring appropriate management and improved outcomes. Given the exceptional rarity of hip involvement as the first clinical indicator of hemophilia, further case reporting and experience from different centers will be necessary to validate our observations and support stronger clinical guidance in similar scenarios.
This case study illustrates the critical importance of considering rare hematological disorders, such as hemophilia, in the differential diagnosis of hip effusion in infants with abnormalities in hemostasis parameters. It highlights those atypical presentations, such as intra-articular bleeding mimicking septic arthritis, can lead to delays in diagnosis and treatment if not recognized. The findings emphasize that thorough laboratory evaluations, including assessments of coagulation factors, are essential to avoid unnecessary invasive procedures.
References
- 1. Taylor GR, Clarke NM. Management of irritable hip: A review of hospital admission policy. Arch Dis Child 1994;71:59-63. [Google Scholar] [PubMed]
- 2. Liberman B, Herman A, Schindler A, Sherr-Lurie N, Ganel A, Givon U. The value of hip aspiration in pediatric transient synovitis. J Pediatr Orthop 2013;33:124-7. [Google Scholar] [PubMed]
- 3. Bolton-Maggs PH, Pasi KJ. Haemophilias A and B. Lancet 2003;361:1801-9. [Google Scholar] [PubMed]
- 4. UKHCDO. Annual Report. Bleeding Disorder Statistics for 2014/2015. Available from: https://www.ukhcdo.org/wpcontent/uploads/2019/04/2015_ukhcdo_annual_report_2014_15_data.pdf(20)(Last accessed date; 23 December 2025) [Google Scholar] [PubMed]
- 5. Rodríguez-Merchán EC. Effects of hemophilia on articulations of children and adults. Clin Orthop Relat Res 1996;328:7-13. [Google Scholar] [PubMed]
- 6. Kulkarni R, Presley RJ, Lusher JM, Shapiro AD, Gill JC, Manco-Johnson M, et al. Complications of haemophilia in babies (first two years of life): A report from the centers for disease control and prevention universal data collection system. Haemophilia 2017;23:207-14. [Google Scholar] [PubMed]
- 7. Berntorp E, Fischer K, Hart DP, Mancuso ME, Stephensen D, Shapiro AD, et al. Haemophilia. Nat Rev Dis Primers 2021;7:45. [Google Scholar] [PubMed]
- 8. Mannucci PM, Tuddenham EG. The hemophilias–from royal genes to gene therapy. N Engl J Med 2001;344:1773-9. [Google Scholar] [PubMed]
- 9. Gualtierotti R, Solimeno LP, Peyvandi F. Hemophilic arthropathy: Current knowledge and future perspectives. J Thromb Haemost 2021;19:2112-21. [Google Scholar] [PubMed]
- 10. Pettersson H, Wingstrand H, Thambert C, Nilsson IM, Jonsson K. Legg-Calvé-Perthes disease in hemophilia: İncidence and etiologic considerations. J Pediatr Orthop 1990;10:28-32. [Google Scholar] [PubMed]
- 11. Van Vulpen LF, Van Meegeren ME, Roosendaal G, Jansen NW, Van Laar JM, Schutgens RE, et al. Biochemical markers of joint tissue damage increase shortly after a joint bleed; an explorative human and canine in vivo study. Osteoarthritis Cartilage 2015;23:63-9. [Google Scholar] [PubMed]
- 12. Floman Y, Niska M. Dislocation of the hip joint complicating repeated hemarthrosis in hemophilia. J Pediatr Orthop 1983;3:99-100. [Google Scholar] [PubMed]
- 13. Trueta J. Studies on the etiopathology of osteoarthritis of the hip. Clin Orthop Relat Res 1963;31:7-19. [Google Scholar] [PubMed]
- 14. Wallny T, Brackmann HH, Seuser A, Diedrich O, Kraft CN. Haemophilic arthropathy of the hip in children–prognosis and long-term follow-up. Haemophilia 2003;9:197-201. [Google Scholar] [PubMed]
- 15. Mubarak SJ, Bialik V. Pavlik: The man and his method. J Pediatr Orthop 2003;23:342-6. [Google Scholar] [PubMed]







