[box type=”bio”] What to Learn from this Article?[/box]
The use of heterologous bone grafts having specific biological and physical properties can be particularly useful for solving complex cases of long-bone recalcitrant non-unions such as the one described here.
Case Report | Volume 7 | Issue 6 | JOCR November – December 2017 | Page 31-35| Ferdinando Da Rin De Lorenzo. DOI: 10.13107/jocr.2250-0685.938
Authors: Ferdinando Da Rin De Lorenzo [1]
[1] Department of Orthopaedics, Institute Codivilla-Putti, Via Codivilla, 132043 Cortina d’Ampezzo, Belluno. Italy.
Address of Correspondence:
Prof. Ferdinando Da Rin De Lorenzo,
Institute Codivilla-Putti, Via Codivilla, 1 32043 Cortina d’Ampezzo, Belluno, Italy.
E-mail: darin.ferdinando@iol.it
Abstract
Introduction: Non-unions at forearms are usually challenging and difficult to treat. If additionally, an infection is present, reconstructive surgery should be planned only after full debridement, antibiotic treatment, and confirmation, based on clinical observation and laboratory tests that the infection has subsided. Bone grafting may be required for reconstruction. The use of autogenous bone calls for a second surgical site with an increased risk of morbidity. Using bone substitutes may reduce the need for autogenous bone. Stimulating factors, such as bone marrow concentrate (BMC) and demineralized bone matrix (DBM), may be used concomitantly with bone substitutes to facilitate bone regeneration.
Case Report: The present report describes the case of a 38-year-old patient whose radius was fractured in a car accident. A first surgery involved stabilizing the reduced fracture with a plate, but an infection developed, and the bone did not heal. 3 months later, a second surgery followed, involving placing an antibiotic-filled spacer. This did not cure the infection, so the spacer was replaced 3 months later, and a second antibiotic was added. The patient also began taking oral antibiotics. 6 months later, the patient underwent vascularized fibular grafting. However, the graft did not integrate, and a non-union developed. A year later, the non-union was treated by grafting autogenous bone from the iliac crest, equine bone substitute, and equine DBM, in conjunction with autologous BMC and platelet-rich plasma. At the 6-month follow-up, the bone structure appeared to be successfully reconstructed.
Conclusion: A graft made of a combination of materials with both biological and physical properties can be used to foster bone regeneration for the treatment of particularly challenging cases of non-unions.
Keywords: Radius, infected non-union, equine demineralized bone matrix, bone marrow aspirate, platelet-rich plasma.
Introduction
After the introduction of dynamic compression plates, non-union of diaphyseal forearm fractures has become relatively uncommon. They develop mainly when technical problems arise (a weak plate, plate misplacement) or an infection develops [1]. A bone defect is usually observed, and treatment calls for grafting autogenous bone or, especially for the radius, a vascularized bone graft [2]. Yet, to overcome the drawbacks related to the use of autogenous bone, the use of bone substitutes may be advisable. In the past few years, an enzyme-treated, collagen-preserving, and equine-derived bone graft have been widely used in oral surgery [3, 4, 5, 6] and orthopedic applications [7]. The native collagen conformation may allow the material to maintain mechanical resistance to compression and shaping [7] and, favoring cell adhesion, and facilitate bone regeneration [8]. In addition, bone regeneration may be stimulated by adding demineralized bone matrix (DBM) [9] and bone-marrow aspirate or concentrate (bone marrow aspirate [BMA]/bone marrow concentrate [BMC]) to the graft [10]. The present report describes a case in which autogenous bone and equine bone grafts and DBM, in addition to autogenous BMC and platelet-rich plasma (PRP), were successfully used in treating a recalcitrant, post-infective non-union of a radius diaphyseal fracture.
Case Report
The 38-year-old male patient was in a car accident in January 2007 and fractured his right radius. The fracture was exposed (Gustilo Type 2). Before presenting at the Codivilla-Putti Hospital in September 2007, his medical records show that, after initial trauma care, he was subjected to immobilization using a cast and that his fracture was reduced and fixated with a plate and screw system (brand unknown) and bone grafts (unknown) in March 2007. Bone grafting was performed because bone loss occurred following the traumatic fracture. His medical records also indicated that in May 2007 he had suffered from a wound dehiscence that was negative for infection (it is not known whether the patient underwent antibiotic therapy). The next month, the plate and screws were removed, and an external fixation system (brand unknown) was placed.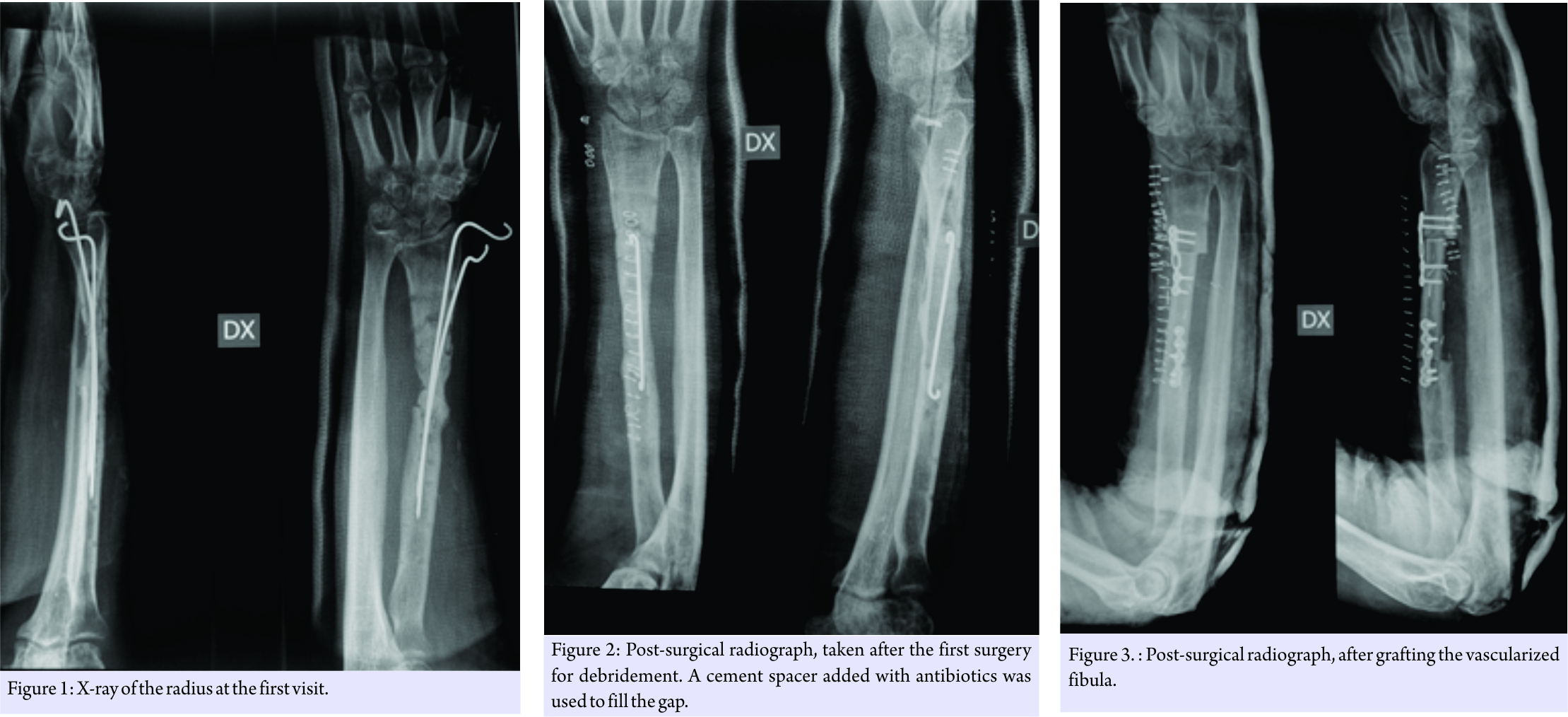
He presented at the Codivilla Hospital complaining of pain and presenting a non-suppurating fistula at the scar site of the previous operations. A radiograph was taken and showed a non-union and bone loss (Fig. 1). In November 2007, the patient underwent surgery aimed at completely debriding the site. A cement spacer containing gentamicin and clindamycin (Copal G+C, Heraeus, Hanau, Germany), along with vancomycin, was placed into the gap (Fig. 2). At this point, the surgical site was positive for Staphylococcus aureus. 3 months later, in February 2008, as a fistula developed again, the spacer was replaced with a new one (same source as the previous surgery). The antibiotic therapy was interrupted 2 weeks before spacer replacement for allowing bacteria strain identification. Before spacer replacement, a white blood cell scintigraphy, laboratory tests for inflammation, including erythrocyte sedimentation rate, C-reactive protein, and complete blood count were performed and radiography was taken. Based on the results of antibiograms, imipenem and vancomycin also were added, and oral antibiotics were also prescribed (ciprofloxacin, 750 mg twice a day). 3 months later, in May 2008, the fistula had disappeared, and the patient’s leukocyte scintigraphy was negative. After 3 more months, in August 2008, the patient underwent reconstruction using a vascularized fibula graft (Fig. 3). At the subsequent control appointments, radiographs showed non-union of the graft (Fig. 4). 8 months later, in April 2009, the graft and the osteosynthesis devices that had been concomitantly placed were removed, and a third spacer was positioned.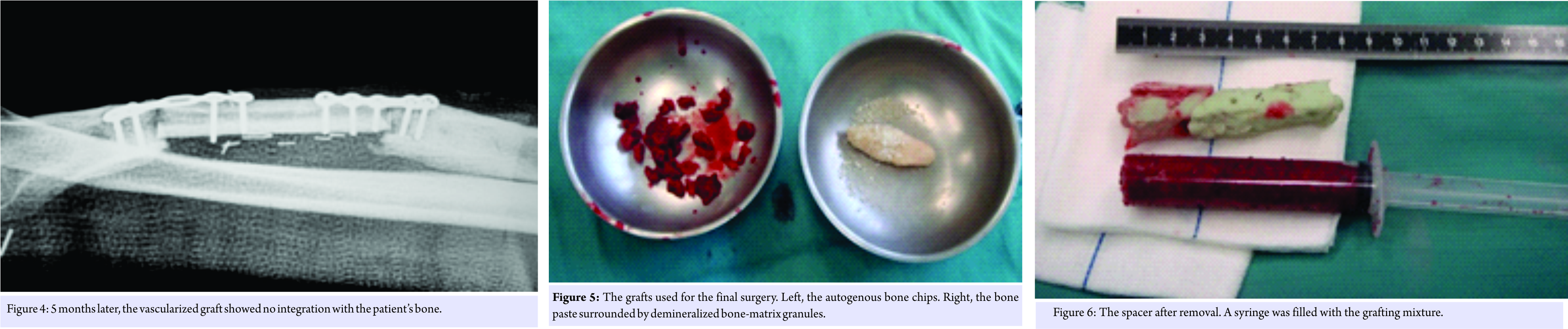
In July 2009, the patient underwent a final surgery to place autogenous bone from the iliac crest; equine bone paste containing equine DBM (Osteoplant Activagen Mouldable Paste, Bioteck, Vicenza, Italy); equine DBM granules (Osteoplant Activagen, Bioteck); autologous BMC obtained during the surgery using a dedicated system (Marrowstim, Biomet, Warsaw, USA); and autologous PRP previously prepared according to standard protocols by the hospital blood transfusion service. Very briefly, the PRP was prepared from a sample of the patient’s blood, using differential centrifugation to separate PRP from platelet-poor plasma and red blood cells. This process increased the platelet concentration approximately four- to five-fold compared to the initial blood sample. Before the application, platelets were activated with thrombin and calcium gluconate. The equine bone paste is a mixture of equine cancellous bone granules (1–2 mm), equine DBM granules (1–2 mm), equine collagen, equine bone powder (<0.2 mm), and water-based gel. The equine bone components are made non-antigenic through an enzyme-based processing method that leaves bone collagen unaltered [8]. The concentration of the different components is adjusted to make the paste easily moldable. Equine DBM granules are manufactured by applying the same process as that used for equine bone and then subjecting the granules to complete acid demineralization.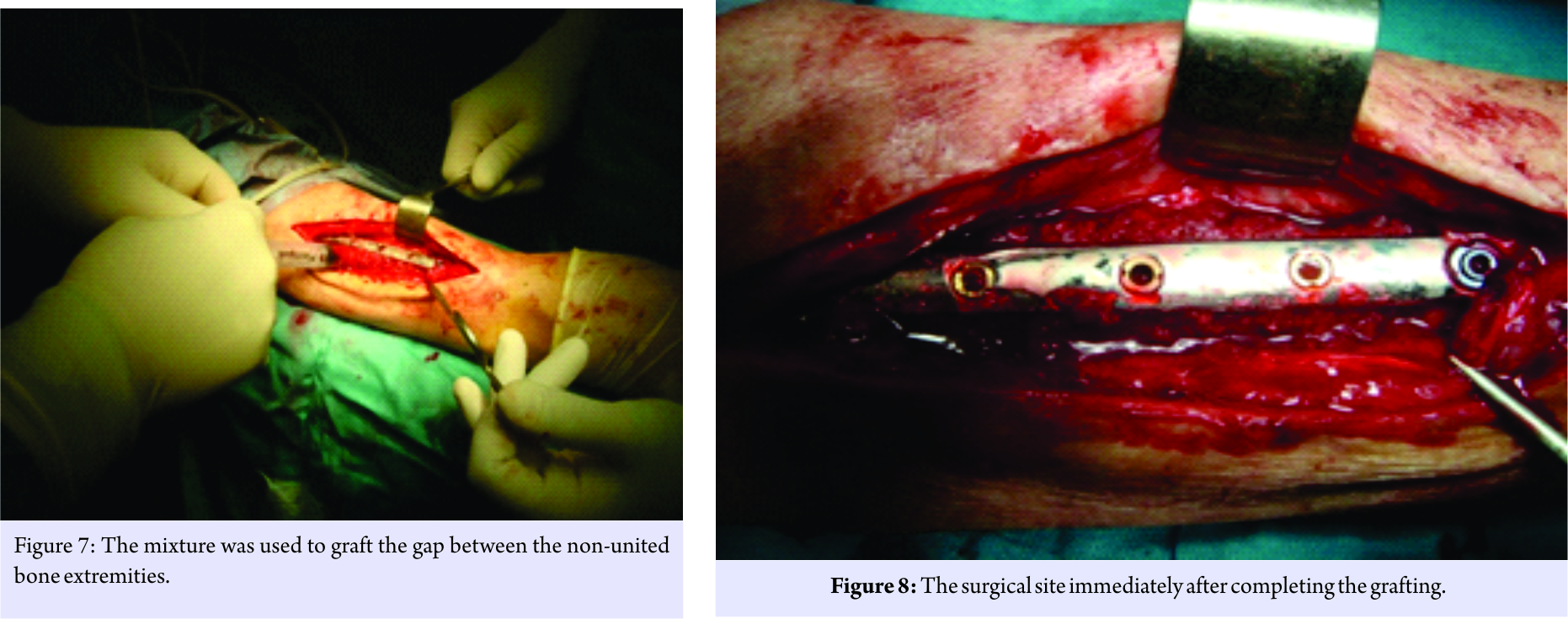
On the day of the surgery, the patient underwent general anesthesia, and two operatory fields were prepared, one at the right iliac crest and one at the right radius. After gaining proper access to the iliac crest through a small incision and removing part of the cortical bone, autogenous cancellous chips were collected using a surgical spoon. Approximately 60 cc of bone-marrow aspirate was collected from the same iliac access, inserting a 5-hole biopsy needle in different directions to optimize the collection of mesenchymal cells. The chips (10 cc), equine DBM (2 cc), equine bone paste (5 cc), BMC (6 cc), and PRP (4 cc) were carefully mixed in a bowel (Fig. 5). A small amount of calcium gluconate was added to activate the PRP and induce clotting. The mixture was then placed in a pre-cut sterile syringe to shape it, after clotting, as a compact cylinder.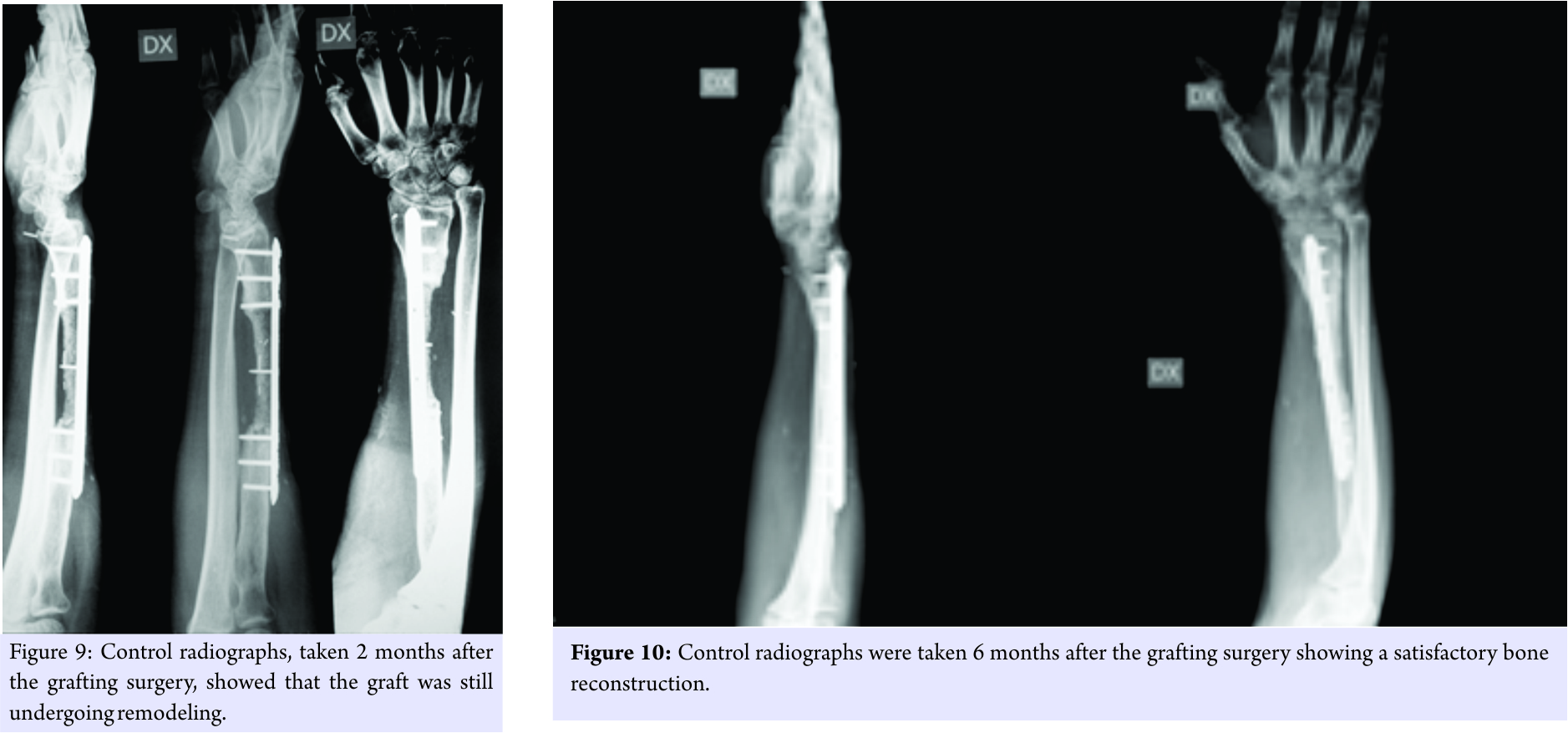
The surgical site at the radius was opened, and the spacer was removed (Fig. 6). The two bone extremities were stabilized with a metal fixation plate, and the approximately 7.5-cm-long gap was filled with the mixture, extruded from the pre-cut syringe (Fig. 7 and 8). At 60 days, radiographs (Fig. 9) showed that the graft was still undergoing remodeling. However, at 6 months, radiographs (Fig. 10) showed that the graft had undergone significant remodeling; its radiolucency had increased significantly.
Discussion
Recalcitrant non-unions of the diaphyseal radius are rare (about 5%) [11] but can be severely disabling. Causes are multifactorial, but infections may certainly impair the ability of bone to regenerate. The case under examination had a long medical history. In the beginning, possible mistreatment may have resulted in the development of both infection and non-union. The vascularized graft may have failed due to a still-latent infection. The success of the final regeneration may be attributable to the use of several types of graft materials, with each adding different osteogenic factors to the graft site. Autogenous bone, in fact, contains differentiated cells that are already capable of starting new bone deposition. BMA delivers a high concentration of mesenchymal stem cells [10]. Although the concentration strongly depends on the preparation method and the patient’s initial hematologic conditions [12], PRP typically provides a whole set of pro-osteogenic growth factors, including transforming growth factor-β1, platelet-derived growth factor, and vascular endothelial growth factor. DBM contains a small but still active concentration of other osteogenic growth factors, such as bone morphogenetic proteins 2 and 7 (BMP2 and 7). In the present case, equine DBM also was used. As BMPs are highly conserved proteins, their amino acid sequences vary only slightly among mammal species, with the protein of one species being capable of stimulating the bone tissue of other species [13]. Therefore, even the equine DBM used in the present case may have contributed to increasing the osteogenic potential at the graft site. DBM also increases the concentration of exposed bone collagen at graft sites, which may also positively affect bone regeneration, as collagen modulates many processes underlying bone formation and remodeling. The equine graft paste enabled the creation of a moldable mixture that was easy to handle and position. It also exerted an additional osteoconductive effect resulting from the bone granules it contains. At present, debate still exists about the best choice of bone-graft material for diaphyseal radius non-unions. Some authors prefer using autogenous cortico-cancellous bone for defects up to 6 cm; they resort to vascularized grafts for longer defects [1]. To reduce patient discomfort and the risk of morbidity, one of the aims of regenerative medicine is to create a condition at the graft site that limits or even eliminates the need for autogenous bone. More investigations are needed, under controlled conditions, to study the effectiveness of pro-regenerative combinations like the one used in the present case.
Conclusion
Conventional treatments may not be enough to treat particularly challenging cases of non-unions. In such cases, to promote bone regeneration and healing, it may be useful to graft the defect with a mixture of components that have biological effects and physical properties that make the graft material easy to handle and position.
Clinical Message
Traditional options for the treatment of long bone recalcitrant non-unions might not allow for adequate bone healing in severe cases, which may instead be successfully resolved using a combination of materials with biological and physical properties that foster bone regeneration and healing.
References
1. Kloen P, Buijze GA, Ring D. Management of forearm nonunions: Current concepts. Strategies Trauma Limb Reconstr 2012;7:1-11.
2. Safoury Y. Free vascularized fibula for the treatment of traumatic bone defects and nonunion of the forearm bones. J Hand Surg Br 2005;30:67-72.
3. Di Stefano DA, Gastaldi G, Vinci R, Polizzi EM, Cinci L, Pieri L, et al. Bone formation following sinus augmentation with an equine-derived bone graft: A retrospective histologic and histomorphometric study with 36-month follow-up. Int J Oral Maxillofac Implants 2016;31:406-12.
4. Di Stefano DA, Gastaldi G, Vinci R, Cinci L, Pieri L, Gherlone E, et al. Histomorphometric comparison of enzyme-deantigenic equine bone and anorganic bovine bone in sinus augmentation: A Randomized clinical trial with 3-year follow-up. Int J Oral Maxillofac Implants 2015;30:1161-7.
5. Artese L, Piattelli A, Di Stefano DA, Piccirilli M, Pagnutti S, D’Alimonte E, et al. Sinus lifts with autologous bone alone or in addition to equine bone: An immunohistochemical study in man. Implant Dent 2011;20:383-8.
6. Pistilli R, Signorini L, Pisacane A, Lizio G, Felice P. Case of severe bone atrophy of the posterior maxilla rehabilitated with blocks of equine origin bone: Histological results. Implant Dent 2013;22:8-15.
7. Santini S, Barbera P, Modena M, Schiavon R, Bonato M. Equine-derived bone substitutes in orthopedics and traumatology: Authors’ experience. Minerva Chir 2011;66:63-72.
8. Perrotti V, Nicholls BM, Piattelli A. Human osteoclast formation and activity on an equine spongy bone substitute. Clin Oral Implants Res 2009;20:17-23.
9. Gruskin E, Doll BA, Futrell FW, Schmitz JP, Hollinger JO. Demineralized bone matrix in bone repair: History and use. Adv Drug Deliv Rev 2012;64:1063-77.
10. Block JE. The role and effectiveness of bone marrow in osseous regeneration. Med Hypotheses 2005;65:740-7.
11. Kloen P, Wiggers JK, Buijze GA. Treatment of diaphyseal non-unions of the ulna and radius. Arch Orthop Trauma Surg 2010;130:1439-45.
12. Magalon J, Bausset O, Serratrice N, Giraudo L, Aboudou H, Veran J, et al. Characterization and comparison of 5 platelet-rich plasma preparations in a single-donor model. Arthroscopy 2014;30:629-38.
13. Scarfì S. Use of bone morphogenetic proteins in mesenchymal stem cell stimulation of cartilage and bone repair. World J Stem Cells 2016;8:1-2.
 |
| Prof. Ferdinando Da Rin De Lorenzo |
| How to Cite This Article: Da Rin De Lorenzo F. Treating a Recalcitrant Non-union of the Radius Using Autogenous Bone, Equine Bone Paste, Equine Demineralized Bone Matrix, Platelet Rich Plasma and Bone Marrow Aspirate. Journal of Orthopaedic Case Reports 2017 Nov-Dec; 7(6): 31-35 |
[Full Text HTML] [Full Text PDF] [XML]
[rate_this_page]
Dear Reader, We are very excited about New Features in JOCR. Please do let us know what you think by Clicking on the Sliding “Feedback Form” button on the <<< left of the page or sending a mail to us at editor.jocr@gmail.com