[box type=”bio”] Learning Point of the Article: [/box]
In this article, we hope readers to learn the clinical manifestations, imaging characters, and diagnosis and treatments of cervical intradural discherniation.
Case Report | Volume 9 | Issue 2 | JOCR March – April 2019 | Page 30-33 | Qirui Ding, Lipeng Yu. DOI: 10.13107/jocr.2250-0685.1356
Authors: Qirui Ding[1], Lipeng Yu[1]
[1]Department of Orthopedic, First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu, China.
Address of Correspondence:
Dr. Lipeng Yu,
Department of Orthopedic, First Affiliated Hospital of Nanjing Medical University, 300 Guangzhou Road, Nanjing, Jiangsu 210000, P.R. China.
E-mail: doctording@126.com; yulipeng@hotmail.com
Abstract
Introduction: Introduction: Intradural disc herniation is a rare disease and it accounts for 0.26–0.30% of all herniated discs. Little was known about intradural disc herniation in the previous studies.
Case Report: Here, we report a 49-year-old male patient with Brown-Sequard syndrome caused by spontaneous cervical intradural disc herniation at C6–C7 level.
Conclusions: It is difficult to be diagnosed before the surgery through computed tomography scans, myelograms, and magnetic resonance image scans. Once it was diagnosed, an operation should be performed.
Keywords: Orthopaedic, case report, spine.
Introduction
Intervertebral disc herniation (IDH) is a common spinal disease, but intradural disc herniation is rare. In 1942, Dandy first reported an IDH case in the lumbar spine [1]. The first cervical IDH (CIDH) and the first thoracic IDH were reported by Marega [2], in 1959, and Chowdhary [3], in 1987, respectively. As of the current reported IDH cases, 3% were found in the cervical, 5% in the thoracic, and 92% in the lumbar region [4, 5].
Case Report
History and neurological examination
A 49-year-old man was transferred to our spinal department, with a 7-day history of weakness in the left upper and lower limbs, accompanied by the right somatosensory deficits. He had undergone a right lobectomy due to a pulmonary adenocarcinoma 4 months ago. A week ago, he suddenly presented a Brown-Sequard syndrome (BSS) while receiving radiotherapy, without any injury or trauma. Sensation to pain, temperature, and slight touch were reduced below the nipple level on the right, while proprioception was reduced on the left. Rectal examination was normal without saddle anesthesia. Muscle strength on the left was 3/5 in the hand, and 4/5 in the quadriceps and ankle dorsiflexion and plantar flexion. Ankle reflex was absent on the left. Neurological examination in the right upper limb was normal.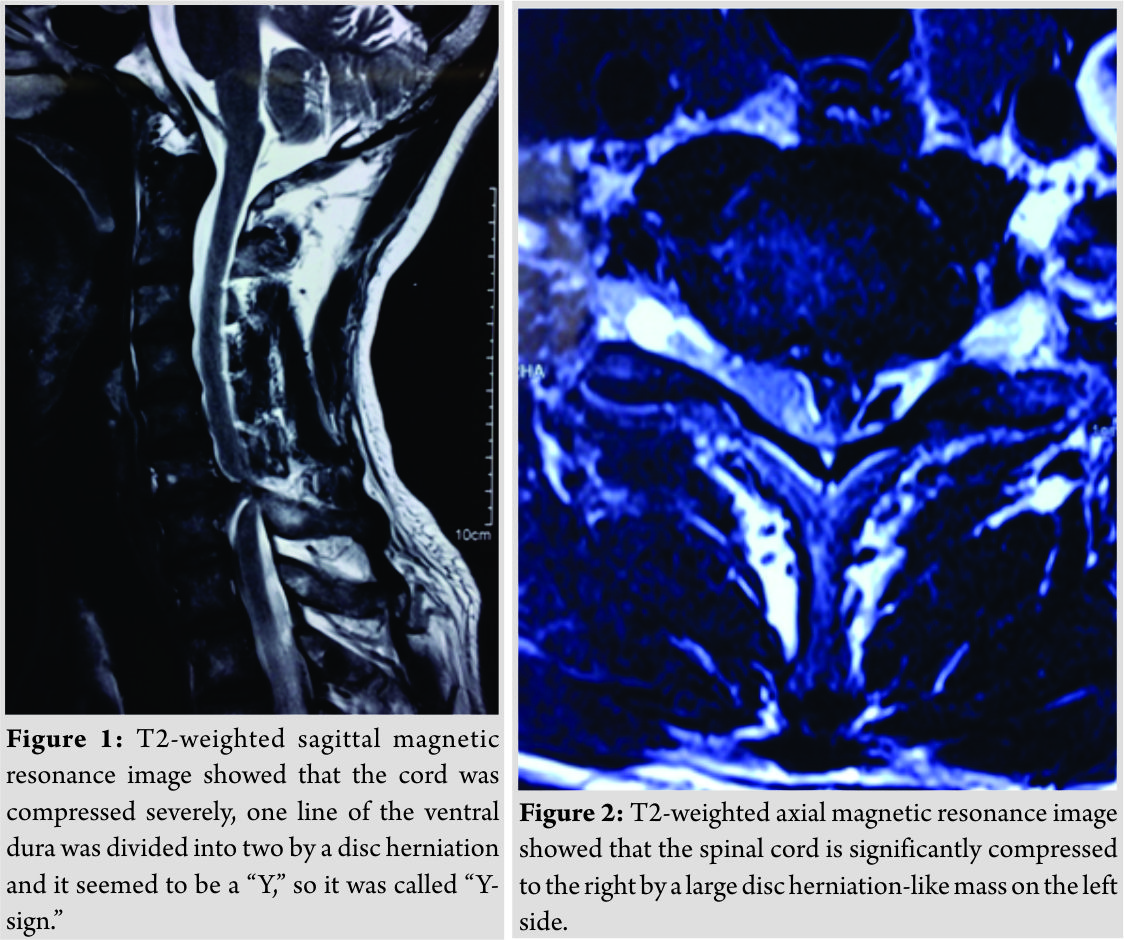
Imaging and laboratory investigations
T2-weighted sagittal magnetic resonance image (MRI) showed a C6–C7 herniated disc compressing the cord and we could see the “Y-sign” (Fig. 1)described by Sasaji et al. [6], which meant that one line of the ventral dura was divided into two by a disc herniation and it seemed to be a “Y.” T2-weighted axial MRI showed that the spinal cord is significantly compressed to the right by a large disc herniation-like mass on the left side (Fig. 2). The mass did not present contrast enhancement in the gadolinium-enhanced MRI (Fig. 3).Computed tomography (CT) scans showed no bony destruction in the cervical spine. The levels of commonly used serum tumor markers were within the normal range.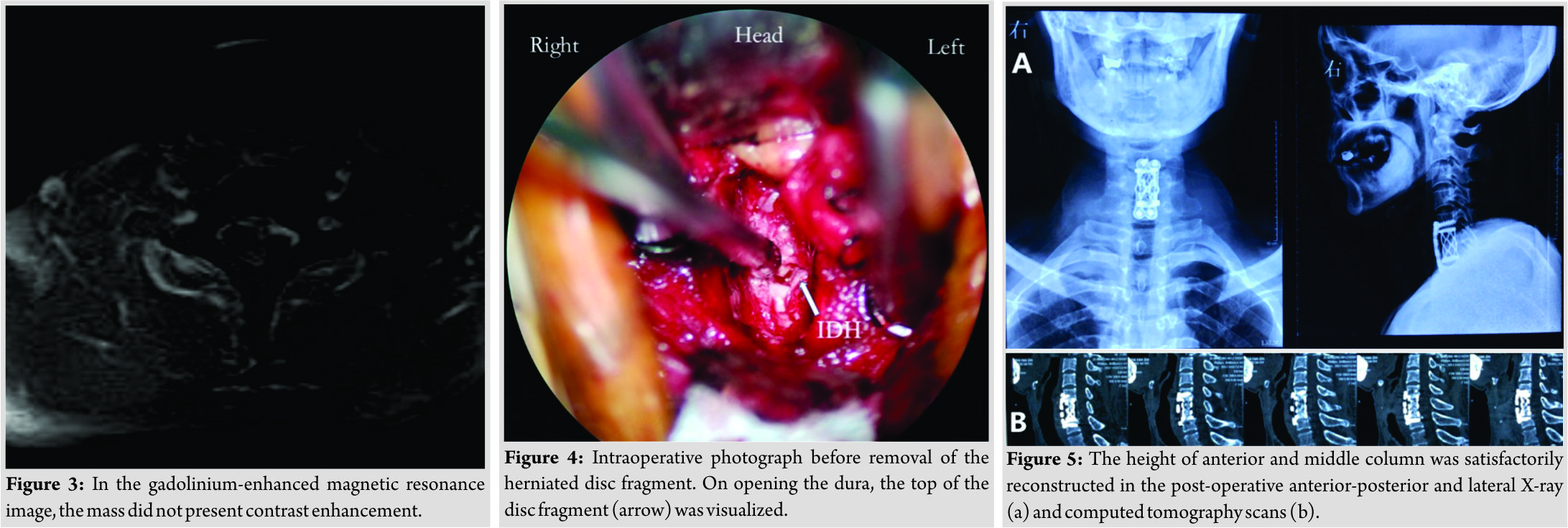
Surgical procedure
We performed an anterior C6/7 discectomy of the patient during operation at first. Part of the extradural disc was removed in the spinal canal, the volume of which was not consistent with the image on the MRI scans. Subsequently, a C6 corpectomy was performed, but no more disc was detected, either. We suspected an intradural herniation. With the help of the operation microscope, we observed a slight bulge adjacent to the upper margin of C7 and part of posterior longitudinal ligament tightly adhered to the dura sac. After opening the dura, larger pieces of nucleus pulpous were exposed (Fig. 4), with cerebrospinal fluid (CSF) leakage, and then, we clamped out the mass. The size of the disc fragment was approximately 2 cm×1 cm×1 cm. Since the tear of the dura was hard to be sutured, we fixed it with artificial dura patch (Tianyifu Medical Treatment Equipment Co., Ltd., Beijing, China). Afterward, a suitable interbody fusion cage (WEGO, Shandong, China) fully filled with autografts and a cervical plate (WEGO, Shandong, China) was properly placed to reconstruct the stability of the cervical spine.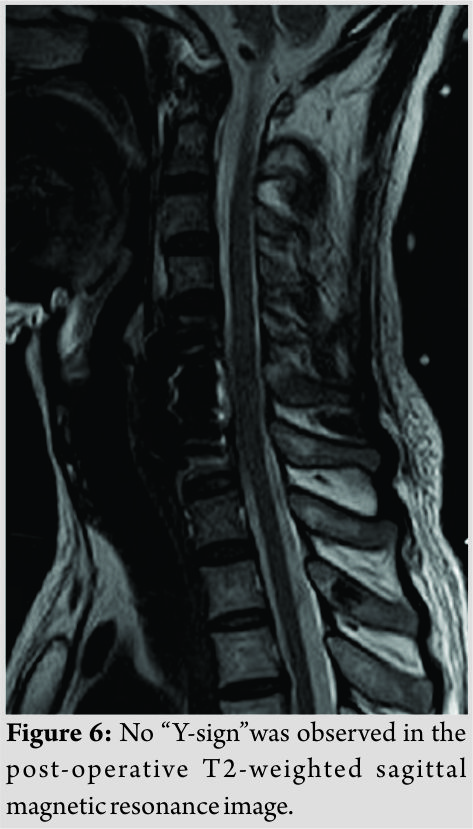
Post-operative treatment and outcome
We performed a CSF lumbar drainage after operation to reduce the CSF leakage in the neck. The muscle strength of the left upper and lower limbs of the patient was completely recovered to 5/5 immediately after surgery, while sensory deficits on the right side of the body existed for 3 months. Pathological analysis of the removed fragments confirmed fibrocartilaginous tissue and degenerative disc diagnosis. The height of anterior and middle columns was restored on the post-operative plain radiographs and CT scans (Fig.5). No “Y-sign” was observed on the post-operative T2-weighted sagittal MRI any more (Fig. 6). The patient proposed no complaint during the post-operative follow-up.
Discussion
The pathogenesis of IDH remains uncertain. Trauma may be a possible explanation proposed by previous authors. However, to the best of our knowledge in literature, only 44.4% of the patients suffered from CIDH had a definite traumatic event [7]. In 2014, the first case of spontaneous CIDH was reported by Warade [8]. In our case, the patient suddenly suffered from a spontaneous CIDH, presenting with a BSS symptom while receiving a radiotherapy, without any history of cervical trauma or injury. The clinical manifestations of spontaneous CIDH vary, depending on the location of IDH. When the IDH compressed the cord ventrally, patients may present with quadriparesis. When compressing the cord laterally, patients may present with complete or incomplete BSS, accompany by Horner’s syndrome (HS) in some patients [8].If only the nerve root is compressed, patients may merely present with radiculopathy. A system review performed by Guan et al. [9] uncovered that the most common level involved was C5–C6, followed by C6–C7, C4–C5, and C3–C4. Typical symptoms include BSS, quadriparesis, and radiculopathy. Baudracco and Grahovac [10] summarized nine cases of spontaneous CIDH before 2017, all patients experienced BSS. In our case, the patient also suffered from complete BSS. It is hard to diagnose the disease of CIDH definitively before surgery. MRI is the most important auxiliary diagnostic technique, on which we can find cord compression, stenosis of spinal canal, extramedullary heterogeneous soft tissue mass, and the “Y-sign” on T2-weighted imaging, as that we had observed in this case. On the enhanced MRI, no obvious contrast enhancement was observed. Sometimes, CT scans, myelography, and X-ray can provide indications. In some CIDH cases, we may see ossification of posterior longitudinal ligament on CT scans [11] or intradural complete or incomplete filling defect, subarachnoid mass lesion displacing the cord contralaterally, widening of the spinal cord in myelography and disc space narrowing, and bone spur on X-ray [9]. However, what we discussed above are indirect diagnostic strategies. Definitive diagnosis needs to be confirmed during the operation, with post-operative pathological analysis. In most cases, no obvious abnormality was observed in blood investigations, especially in the tumor marker examination. Once CIDH is highly suspected, surgery is the first option. Of all the CIDH cases reported, up to now, anterior approach surgeries, including anterior cervical corpectomy with fusion (ACCF) and anterior cervical discectomy with fusion (ACDF), were performed more frequently than posterior approach [8]. IDH originates from intervertebral disc, disc herniation usually residues in the ventral extradural space. Extra- and intra-dural disc herniations can be removed completely at the same time with ACCF or ACDF. In some of the reported cases, posterior approach surgeries were performed since the IDHs transmigrate to the back and compress the dorsal cord. Microscope is recommended when performing durotomy, which can minimize the damage to the cord. With the help of a microscope, the incision of dura can be sutured carefully to prevent post-operative CSF leakage. In our case, the suture of the incision was difficult, so an artificial dura patch was applied to cover the breakage. However, we performed a continuous lumbar CSF drainage for a week to decrease the pressure of dura sac and prevent CSF leakage into the cervical anterior space. No obvious CSF leakage after operation was detected, but the necessity of lumbar CSF drainage remains a discussion in literature. After the operation, all the reported cases achieved different degree of recoveries of the neurological function. Some patients gained full recoveries, but for most patients, more or less, residual symptoms remained. Motor disorders recovered immediately in most cases, whereas sensory disorders existed for a long period. In our case, the muscle strength of the patient was completely recovered immediately after surgery, while sensory deficits existed for 3 months.
Conclusion
Spontaneous CIDH is a rare event which can cause compression of spinal cord and nerve root, result in corresponding clinical symptoms such as radiculopathy, BSS, and HS. Nevertheless, it remains difficult to be definitively diagnosed preoperatively. Some indirect signs on the MRI such as the “Y sign” can provide us with indications of CIDH. Anterior corpectomy and durotomy under a microscope are the first option as recommended and posterior approach surgery is required when necessary. Surgical treatment usually yields good clinical outcome and complete recovery. The pathogenesis of IDH remains to be further investigated.
Clinical Message
Intradural disc herniation (IDH) is a rare disease, especially in the cervical spine. We present a case of spontaneous CIDH at C6–C7 level causing Brown-Sequard syndrome. We want to share the experience of how to diagnose and treat CIDH with other orthopedists all over the world.
References
1. Dandy WE. Serious complications of the ruptured intervertebral disks. JAMA 1942;119:474-7.
2. Marega T. Injury and hernia of the disk. Minerva Ortop 1959;10:649-52.
3. Chowdhary UM. Intradural thoracic disc protrusion. Spine (Phila Pa 1976) 1987;12:718-9.
4. Epstein NE, Syrquin MS, Epstein JA, Decker RE. Intradural disc herniations in the cervical, thoracic, and lumbar spine: Report of three cases and review of the literature. J Spinal Disord 1990;3:396-403.
5. Negovetic L, Cerina V, Sajko T, Glavić Z. Intradural disc herniation at the T1–T2 level. Croat Med J 2001;42:193-5.
6. Sasaji T, Horaguchi K, Yamada N. The specific sagittal magnetic resonance imaging of intradural extra-arachnoid lumbar disc herniation. Case Rep Med 2012;2012:383451.
7. Gunasekaran A, de Los Reyes NK, Walters J. Clinical presentation, diagnosis, and surgical treatment of spontaneous cervical intradural disc herniations: A review of the literature. World Neurosurg 2018;109:275-84.
8. Warade AG. Spontaneous cervical intradural disc herniation. J Clin Neurosci 2014;21:872-3.
9. Guan Q, Xing F, Long Y. Cervical intradural disc herniation: A systematic review. J Clin Neurosci 2018;48:1-6.
10. Baudracco I, Grahovac G. Spontaneous cervical intradural disc herniation presenting with Brown-Séquard and Horner’s syndrome: Lesson learned from a very unique case. Eur Spine J 2017;26:218-21.
11. Wang D, Wang H. Spontaneous cervical intradural disc herniation associated with ossification of posterior longitudinal ligament. Case Rep Orthop 2014;2014:256207.
 |
 |
| Dr. Lipeng Yu |
Dr. Qirui Ding |
| How to Cite This Article: Ding Q, Yu L. Spontaneous Cervical Intradural Disc Herniation Presenting with Brown-Sequard Syndrome at C6–C7 level. Journal of Orthopaedic Case Reports 2019 Mar-Apr; 9(2): 30-33. |
[Full Text HTML] [Full Text PDF] [XML]
[rate_this_page]
Dear Reader, We are very excited about New Features in JOCR. Please do let us know what you think by Clicking on the Sliding “Feedback Form” button on the <<< left of the page or sending a mail to us at editor.jocr@gmail.com




