Implantation of autologous osteoblasts can lead to new bone formation and accelerated healing in non-union fractures.
Dr. Arvind Mehra,
Department of Orthopedics and Trauma, Paras Hospitals, Gurugram, Haryana, India.
E-mail: arvind_mehra@hotmail.com
Introduction: Management of non-union and/or malunited fractures remains a challenge and achieving complete and permanent union with current standard of care is difficult. Treatments that can allow localized and accelerated three-dimensional bone formation can offer an encouraging outcome.
Case Report: We present a case of a middle-aged woman with femoral neck non-union, treated with a novel bone cell therapy. Fracture union was achieved within 75 days post-cell implantation. At 10-month post-cell therapy, clinical outcome in our patient was quite satisfactory and the patient is doing very well without pain or any symptoms. and reports excellent quality of life.
Conclusion: We recommend the use of bone cell therapy that can provide accelerated bone healing and clinical benefits in the short-term significant long-term clinical benefits for the patient.
Keywords: Non-union fracture, femoral neck, bone cell therapy, autologous osteoblasts.
Femoral neck fractures are the most common injuries encountered by older individuals and are associated with high mortality and morbidity. Non-union and avascular necrosis of the femoral head or a combination of both is the main complication following fractures of the femoral neck. Bone non-union after a fracture is a condition associated with high clinical burden in terms of pain and reduced quality of life often with psychological, social, and financial consequences [1]. Internal fixation of femoral neck fracture with cannulated screws placed with a configuration of an inverted triangle remains a feasible and effective treatment [2,3]. Despite improved operative techniques, non-union is still reported in 10–20% of cases [3] and delayed unions in about 30% of neck fractures [4]. The causes of non-union are commonly multifactorial, consisting of biological, mechanical, injury, and patient factors; all contributing to putting a fracture at a risk of nonunion [5].
Mechanical and biological processes are involved in fracture healing and any impairment in these factors can lead to nonunion [1]. Biological factors refer to the local environment at the fracture site, such as the presence of infection, the extent of bone loss, the vascularity of the bone and quality of the surrounding soft tissues, and availability of molecular mediators, progenitor cells and matrix, and immunoregulatory cells among others [6,7,8]. Stability of the fracture is contributed by mechanical factors and techniques like mechanical fixation that can help in reducing excess strain. Despite internal fixation in many cases, fractures can end in atrophy due to inadequate biological environment.
Lack of viable biological environment at the site of fracture leads to poor regeneration of bone and results in nonunion. Biological factors refer to the local environment at the fracture, such as the presence of infection, the extent of bone loss, the vascularity of the bone and quality of the surrounding soft tissues, and availability of molecular mediators, progenitor cells, matrix, and immunoregulatory cells [9]. It is necessary to ensure that the biological microenvironment around the fracture is suitable for three-dimensional bone formation that can lead to a functionally repaired bone.
Femoral neck non-unions result in decreased mental and physical health, along with debilitating impacts on activities of daily living [10]. Hence, while treating hip neck fracture patients, it is imperative to monitor for post-operative healing and begin appropriate evaluation for possible non-union for those patients who show any of the above-mentioned symptoms. Failure to recognize nonunion could result in further complications and need for revision surgery in an emergency setting [11,12].
In this case report, we present how timely monitoring of post-operative progress of a femoral neck fracture fixation helped in identifying the non-union type and appropriate bone cell therapy treatment using autologous cultured osteoblast cells led to complete bone union.
A 56-year-old woman reported to our orthopedic hospital with a complaint of severe pain in the left hip and inability to stand or walk. The family reported that the patient had a fall on the same day. Routine investigation revealed a fracture in the neck region of the left femur. There was no history of any comorbid condition. The patient was taken up for a surgical intervention following standard of care compression fixation. The patient’s condition on day 3 post-surgery was satisfactory and the patient was discharged, with recommendation for regular follow-up.
After about 8-month post-discharge, the patient visited our unit complaining of recurring severe pain in the left hip. Radiological investigation revealed a non-union of the previous fracture.
Treatment using a novel bone cell therapy product (OSSGROW®) that is available in the India was considered given the age and lifestyle of the patient. The patient and her family were counseled about the cell therapy option and an appropriate consent was taken.
Osteoblast cell therapy is a two-step process. The first step involves bone marrow harvest and the second step involves implantation of the ex vivo cultured autologous osteoblast cells from the bone marrow. First, an adequate quantity (4–5 ml) of bone marrow from the patient’s iliac crest was aspirated and collected in transport media containing an anticoagulant. The collection kit was transported under temperature-monitored conditions and the collected bone marrow was processed at the GMP-certified cell processing facility (Regrow Biosciences Pvt. Ltd.). Mesenchymal progenitor cells were isolated from the bone marrow of the patient. These progenitor cells were differentiated into bone forming cells or osteoblasts [13]. Immunophenotypic characterization was done to ensure that the cultured cells test positive for osteoblast biomarkers. Osteoblasts were expanded for approximately 4 weeks under stringent laboratory conditions and multiplied to more than 50 million osteoblasts. The personalized autologous osteoblast product OSSGROW® was made available by the 5th week and the cell implantation was planned. In a short surgery, a small incision was made on the lateral cortex of femur shaft to expose the non-union region and the cultured osteoblast cells mixed with a gel (Tisseel kit from Baxter) was injected into the region of non-union.
The patient was advised a rehabilitation protocol to be followed for about 6 weeks. With initial 48 h of immobilization, the patient started with non-weight-bearing exercises; with gradual partial weight-bearing followed by full weight-bearing exercises.
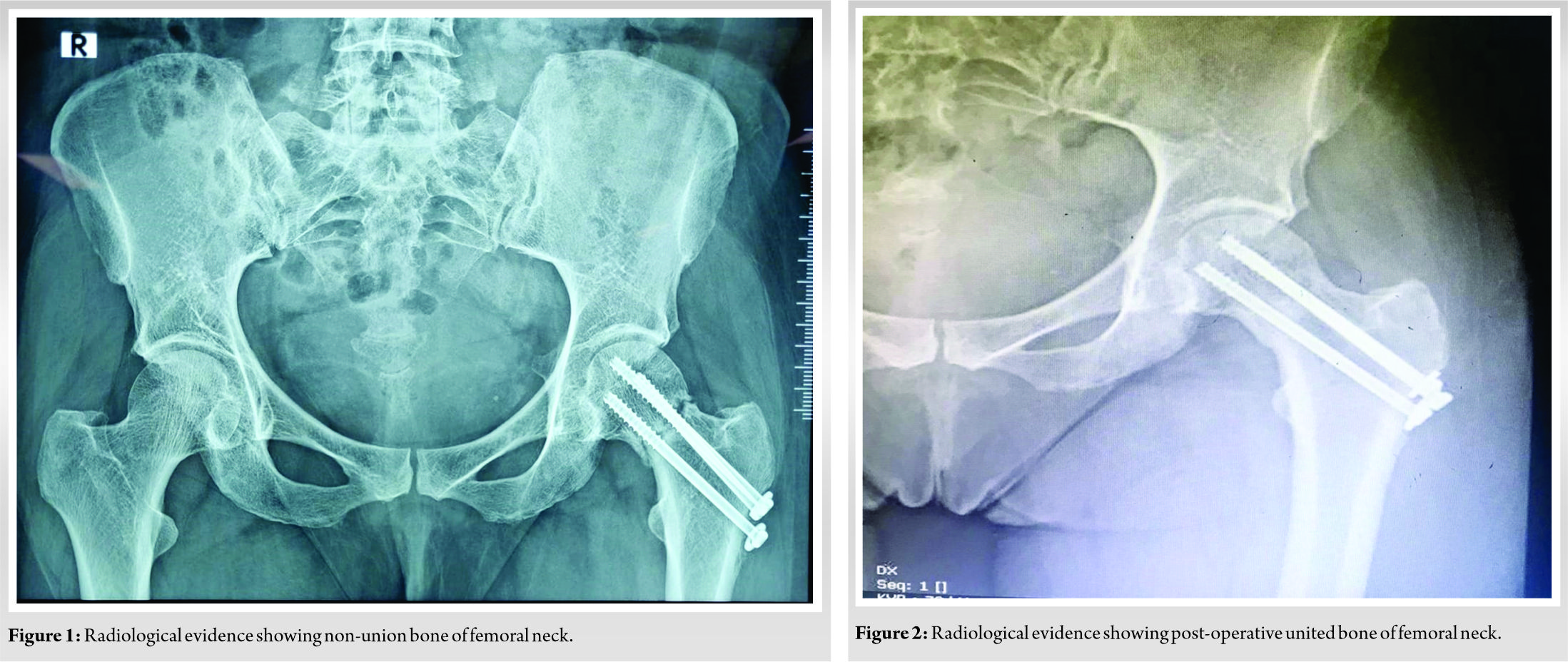
At week 10 post-OSSGROW® treatment, radiological evaluation of the patient showed completely united femoral neck. (Fig. 1) shows radiological evidence of non-union of the fracture in the left hip and (Fig. 2) shows post-operative at 10 weeks with united bone at the femoral neck. The patient had no pain and other symptoms. At 10 months post-implantation, the patient can perform all routine chores, walks without support, and is experiencing very good quality of life.
In this case report, a 56-year-old patient with non-union fracture of the femoral neck was treated successfully with bone cell therapy.
Successful fracture healing requires a viable biological microenvironment and mechanical stability [14]. Fracture healing is a complex biological process involving interactions between mesenchymal stem cells, local inflammatory cytokines, and mechanical stimuli. Healing of bone starts with the inflammation phase where hematoma formation is seen along with tissue formation and proliferation of osteoblasts. This is followed by the repair and remodeling phases where callus and cartilage formation as well as newly woven bone remodeling occur through osteoblastic activity [15].
Fractures with compromised biology do not heal and lead to non-union. The cellular pathology at the non-united ends of bone is characterized by increased osteoclast activity and results in continued loss of bone. The fracture gap slowly widens, and “gold standard” autografts are less likely to give satisfactory results.
Biological substances referred to as “orthobiologics” help in healing musculoskeletal injuries faster [16]. The regenerative potential of orthobiologics is what makes it quite promising especially for tissue repair [17]. Among orthobiologic approaches for bone healing, cell-based therapies and bone marrow derivatives with or without bone grafts and biomaterials have been widely investigated in the recent years [18,19,20]. Orthobiologics have shown to increase cellularity [21]. Fixation procedures augmented with cellular regeneration techniques enhance the probability of clinical improvement. We have used bone cell therapy – autologous ex vivo cultured and expanded osteoblast cell (OSSGROW®) for bone regeneration in the case of non-union of femoral neck fracture. Clinical outcome of complete union was achieved at 10 months post-cell implantation with no pain or other symptoms suggesting that autologous osteoblast treatment can be used for non-union fractures.
In femoral neck fracture cases that end up with either delayed union or non-union, biological treatment with autologous cultured osteoblasts should be considered as a viable option ahead of valgus osteotomy and replacement arthroplasty.
Fractures with compromised biology must be treated with biological solutions that can confer a permanent union of bone. Regeneration of new bone using autologous cultured osteoblasts is a viable biological mode of therapy. Bone remodeling involves osteoblasts, and their recruitment can counter osteoclastic activity. This would help regain balance required for bone remodeling, healthy bone regeneration and effective bone union. Clinical outcome in our patient has been more than satisfactory with complete bone union and the patient is experiencing excellent quality-of-life suggesting that autologous osteoblast cell therapy can be used to achieve bone union in the treatment of non-union fractures.
Using autologous cultured osteoblasts, OSSGROW® is a viable biological mode of cell therapy that can achieve accelerated healing with bone formation leading to bone union in the treatment of non-union fractures..
References
- 1.Stewart SK. Fracture non-union: A review of clinical challenges and future research needs. Malays Orthop J 2019;13:1-10. [Google Scholar | PubMed]
- 2.Guo J, Dong W, Qin S, Zhang Y. Definition of ideal configuration for femoral neck screw fixation in older people. Sci Rep 2019;9:12895. [Google Scholar | PubMed]
- 3.Mathews V, Cabanela ME. Femoral neck nonunion treatment. Clin Orthop Relat Res 2004;419:57-64. [Google Scholar | PubMed]
- 4.Raaymakers EL, Marti RK. Nonunion of the femoral neck: Possibilities and limitations of the various treatment modalities. Indian J Orthop 2008;42:13-21. [Google Scholar | PubMed]
- 5.Lim HS, Kim CK, Park YS, Moon YW, Lim SJ, Kim SM. Factors associated with increased healing time in complete femoral fractures after long-term bisphosphonate therapy. J Bone joint Surg Am 2016;98:1978-87. [Google Scholar | PubMed]
- 6.Loi F, Córdova LA, Pajarinen J, Lin TH, Yao Z, Goodman SB. Inflammation, fracture and bone repair. Bone 2016;86:119-30. [Google Scholar | PubMed]
- 7.Andrzejowski P, Giannoudis PV. The ‘diamond concept’ for long bone non-union management. J Orthop Traumatol 2019;20:21. [Google Scholar | PubMed]
- 8.Kim T, See CW, Li X, Zhu D. Orthopedic implants and devices for bone fractures and defects: Past, present and perspective. Eng Regen 2020;1:6-18. [Google Scholar | PubMed]
- 9.Schlickewei CW, Kleinertz H, Thiesen DM, Mader K, Preimel M, Frosch K, et al. Current and future concepts for the treatment of impaired fracture healing. Int J Mol Sci 2019;20:5805. [Google Scholar | PubMed]
- 10.Brinker MR, O’Connor DP. The biological basis for nonunions. JBJS Rev 2016;4(6):1-9; 01874474-201606000-00001. [Google Scholar | PubMed]
- 11.Brinker MR, Trivedi A, OʼConnor DP. Debilitating effects of femoral nonunion on health-related quality of life. J Orthop Trauma 2017;31:e37-42. [Google Scholar | PubMed]
- 12.Babcock S, Kellam JF. Hip fracture non-unions: Diagnosis, treatment and special considerations in elderly patients. Adv Orthop 2018;2018:1912762. [Google Scholar | PubMed]
- 13.Palekar G, Bhalodiya HP, Archik S, Trivedi K. Retrospective study on implantation of autologous-cultured osteoblasts for the treatment of patients with avascular necrosis of the femoral head. Orthop Res Rev 2021;13:15-23. [Google Scholar | PubMed]
- 14.Virk MS, Lieberman JR. Biologic adjuvants for fracture healing. Arthritis Res Ther 2012;14:225. [Google Scholar | PubMed]
- 15.Einhorn TA, Gerstenfeld LC. Fracture healing: Mechanisms and interventions. Nat Rev Rheumatol 2015;11:45-54. [Google Scholar | PubMed]
- 16.Dhillon MS, Behera P, Patel S, Shetty V. Orthobiologics and platelet rich plasma. Indian J Orthop 2014;48:1-9. [Google Scholar | PubMed]
- 17.Dimitriou R, Jones E, McGonagle D, Giannoudis PV. Bone regeneration: Current concepts and future directions. BMC Med 2011;9:66. [Google Scholar | PubMed]
- 18.Wang W, Yeung K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact Mater 2017;2:224-47. [Google Scholar | PubMed]
- 19.Emara KM, Diab RA, Emara AK. Recent biological trends in management of fracture non-union. World J Orthop 2015;6:623-8. [Google Scholar | PubMed]
- 20.Chang Y, Cho B, Kim S, Kim J. Direct conversion of fibroblasts to osteoblasts as a novel strategy for bone regeneration in elderly individuals. Exp Mol Med 2019;51:1-8. [Google Scholar | PubMed]
- 21.Lin SS, Yeranosian MG. The role of orthobiologics in fracture healing and arthrodesis. Foot Ankle Clin 2016;21:727-37. [Google Scholar | PubMed]