Be careful for the generalized tonic-clonic seizure caused by tranexamic acid administration in the immediate post-operative period.
Dr. Nobuhiro Kaku, Department of Orthopedic Surgery, Faculty of Medicine, Oita University, Yufu City, Oita, Japan. E-mail: nobuhiro@oita-u.ac.jp
Introduction: Tranexamic acid, an antifibrinolytic solvent, has been widely used for perioperative hemostasis in orthopedic surgery. However, to the best of our knowledge, there have been no reports in the literature of seizures caused by tranexamic acid administration for orthopedic surgery. This report presents a case of generalized tonic-clonic seizure caused by tranexamic acid administration immediately after lumbar interbody fusion surgery for lumbar spinal canal stenosis.
Case Report: A 66-year-old Japanese woman scheduled for lumbar interbody fusion surgery was administered an intravenous pre-operative dose (1000 mg) of tranexamic acid and 2000 mg immediately after the surgery. Generalized convulsive seizures occurred on awakening from anesthesia. Although the seizures disappeared after deepening the anesthesia, they recurred on awakening, and extubation could not be performed. A computed tomography scan was immediately performed, revealing an intracranial lesion, but there were no other obvious abnormal findings. The patient was then managed in the intensive care unit, and several convulsions occurred on the 2nd post-operative day. The convulsions disappeared on the 3rd post-operative day, and the patient has had no sequelae to date.
Conclusion: This original case report will be of interest to orthopedic surgeons, anesthesiologists, neurologists, and pharmacologists. The information presented may also have a broader impact in the field of medicine for other types of surgeons. The details provided in the report will advance knowledge in the area of orthopedic surgery, neurology, pharmacology, and anesthesiology. Orthopedic surgeons should be aware that one of the major complications of tranexamic acid administration is seizure liability.
Keywords: Tranexamic acid, seizure, spine surgery, orthopedic surgery.
Many papers in the field of orthopedic surgery have reported tranexamic acid to be useful in reducing blood loss in spine surgery, trauma, and arthroplasty, and the drug is now widely used in the perioperative period [1,2]. Tranexamic acid binds to plasmin and plasminogen, prevents the binding of plasmin and plasminogen to fibrin, and exerts a hemostatic effect through antifibrinolytic action. In contrast, when tranexamic acid crosses the cerebrovascular barrier, it binds to gamma-aminobutyric acid (GABA) receptors and amplifies neuronal excitation without opening the chlorine channels. As a result, generalized tonic-clonic seizures develop [3,4]. Although there have been reports of seizures caused by tranexamic acid administration in cardiovascular surgery [5,6,7], wherein tranexamic acid doses are relatively high, to the best of our knowledge, there have been no reports of seizures caused by tranexamic acid administration in orthopedic surgery. In the present report, we describe a seizure induced by tranexamic acid administration after spinal surgery.
Ethical information
This study was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Written informed consent was obtained from the patient for participation and publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
History of present illness
A 66-year-old Japanese woman (height, 160 cm; weight, 49 kg; and body mass index; 19.1 kg/m2) presented to our hospital with low back and right buttock pain, which first appeared in November 2019. The patient was followed up with conservative therapy; however, the pain gradually worsened and spread to the entire lower limb, and the patient became aware of intermittent claudication. In June 2020, lumbar laminectomy and interbody fusion were scheduled for lumbar spinal canal stenosis.
Medical history
The patient had a history of surgery for the right distal radius fracture and right carpal tunnel syndrome. She took rosuvastatin calcium for dyslipidemia and pregabalin for symptomatic relief of numbness. The patient had a history of smoking 20 cigarettes/day and no drinking history.
Patient examination/imaging
A pre-operative simple radiograph of the lumbar spine (Fig. 1) showed spondylotic changes. Dynamic imaging revealed instability of L2-L3 and narrowing of the intervertebral space at L3-L4 and L4-L5. Magnetic resonance imaging (MRI) of the lumbar spine showed spinal canal stenosis at L2-S1. Lumbar spine myelography revealed dural canal drainage at L2-S1. Respiratory function tests showed mild obstructive impairment with a forced expiratory volume in 1 s of 68.9%. Still, there were no abnormal findings in blood counts, biochemical tests, simple chest radiographs, electrocardiography, or echocardiography. Anesthesia was maintained with 2 L/min oxygen, 2 L/min air, and 1.5% sevoflurane, with 150 μg fentanyl citrate used as needed.
Surgical information
General anesthesia was induced with 100 mg of propofol and 50 μg of fentanyl citrate under oxygen administration. Muscle relaxation was achieved with 30 mg of rocuronium bromide, and intubation was performed. An intravenous tranexamic acid dose of 1,000 mg was administered at the beginning of surgery and 2000 mg immediately after surgery. The surgery was completed without complications (Fig. 2). The operative time was 291 min. The patient’s blood loss was 850 mL, urine volume was 250 mL, and 400 mL of autologous blood was returned as the blood product. The blood test results immediately after surgery were as follows: Blood glucose, 105 mg/dL; sodium, 142 mmol/L; potassium, 4.0 mmol/L; chloride, 110 mmol/L; and calcium, 8.9 mg/dL. After the operation, the patient was placed in the supine position, and a seizure lasting approximately 1 min was observed when the patient was awakened. After resedation with 50 mg propofol, the seizures disappeared. Subsequently, strong seizures were observed on awakening from anesthesia. Blood gas analysis results showed a pCO2 of 15.3 mmHg, HCO3- of 13.9 mEq/L, pH of 7.10, and metabolic acidosis; therefore, 20 mL of sodium bicarbonate was administered. Neuroleptic malignant syndrome was included in the differential diagnosis. A computed tomography (CT) scan of the head (Fig. 3a) was performed to rule out intracranial disease. By the time, the patient was transferred to the intensive care unit, she had a total of 10 seizures, which were treated with diazepam.
Post-operative course/follow-up
The patient was transferred to the intensive care unit of a university hospital and started on continuous intravenous midazolam, fentanyl citrate, and levetiracetam. However, three more seizures were observed on the 1st and 2nd post-operative day. The dose of levetiracetam was increased from 1000 mg/day to 3000 mg/day and the seizures stopped on the 3rd post-operative day. Midazolam was discontinued on the 3rd post-operative day, and fentanyl citrate was discontinued on the 4th post-operative day. Levetiracetam was changed to oral medication and tapered over 12 weeks.
Electroencephalography (EEG) was performed on the 5th post-operative day, but there were no suspicious findings of epileptic seizures. MRI of the head (Fig. 3b) was performed 4 weeks after the surgery. There were no ischemic or hemorrhagic changes in the head that could have caused seizures, and the patient was able to live independently without any sequelae.
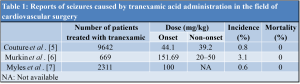
The incidence of post-operative seizures during general anesthesia is very low. Post-operative seizures are the most common complication in neurosurgery, with a reported incidence of approximately 17% [8]; however, the incidence varies from 3% to 92%, depending on the surgical technique. The incidence of seizures after cardiac surgery is 0.1–1.1% [9]. The causes include (1) cerebrovascular lesions (especially cortical lesions) [10], (2) adverse drug reactions, (3) electrolyte abnormalities, and (4) hyperglycemia and hypoglycemia. Cerebrovascular diseases not only include obsolete lesions existing before surgery, but also acute lesions in the perioperative period, such as atheroma, thrombus, embolus due to air release, ischemia (due to uneven reflux in extracorporeal circulation or carotid stenosis), and hemorrhage. The most common adverse drug reaction is the addiction to adrenergic agonists, antidepressants, antipsychotics, and acetylsalicylic acid (aspirin). Hyponatremia and hypocalcemia can occur and are associated with electrolyte abnormalities. In diagnostic strategies for the current patient, EEG, neuroimaging before and after surgery, and blood tests (blood count, electrolytes, and blood glucose) are methods that are generally performed. EEG is particularly useful as a predictor of recurrence risk [11]. In head imaging tests, the sensitivity of CT for cerebral infarction within 6 h of onset is 61%, specificity is 65%, and the inter-reader agreement is also low. However, the sensitivity and specificity for MRI diffusion-weighted imaging are 91% and 95%, respectively [12]. In this case, there were no findings of cerebrovascular disease on post-operative CT performed immediately after the seizure or on subsequent MRI. Hence, cerebrovascular disease is unlikely to have caused the seizures [10]. In addition, in cardiovascular surgery, the surgical field is filled with carbon dioxide during open-heart surgery to prevent microbubbles from entering the heart. The possibility of air bubbles entering the blood vessels is very low in orthopedic surgery. Therefore, it is unlikely that cerebrovascular lesions induce convulsive seizures after surgery. Furthermore, in our patient, pre-operative blood tests showed that electrolyte abnormalities, hyperglycemia, and hypoglycemia were unlikely to occur. There have been reports of tranexamic acid-induced seizures [5,6,7] in cardiovascular surgery, with an incidence of approximately 0.5–3% (Table 1). Tranexamic acid binds to plasmin and plasminogen, prevents its binding to fibrin, and exerts a hemostatic effect via antifibrinolytic action. Aprotinin and epsilon-aminocaproic acid have similar pharmacological effects. Still, the production of aprotinin was discontinued after it was found to cause increased post-operative renal damage, ischemic lesions, and mortality [13] compared to tranexamic acid and epsilon-aminocaproic acid. In contrast, antifibrinolytic therapy with tranexamic acid before cardiopulmonary resuscitation is useful [14]; however, its high-dose administration has been reported to increase the incidence of convulsions after cardiac surgery [7]. This dose is characterized by convulsions in the post-operative period of wakefulness after valve replacement surgery, with no neurological sequelae [7]. The mechanism by which tranexamic acid induces seizures is based on reports of seizures induced by direct cortical or subarachnoid administration of the drug [3]. Among the GABA receptors in the central nervous system, tranexamic acid acts antagonistically on ionic GABAA receptors, which have anxiolytic and anticonvulsant effects. It prevents intracellular Cl ion influx and inhibitory responses due to hyperpolarization. This concentration-dependent excitatory depolarization of neurons and hyperexcitability has been confirmed in animal experiments [4]. In animals, only minimal tranexamic acid crosses the blood-brain barrier. Therefore, although further research is needed to derive a causal relationship between intravenous tranexamic acid and the development of seizures, it is important to note that, in clinical practice, tranexamic acid should be used only in cases with a high risk of massive perioperative bleeding, such as reoperation, long extracorporeal circulation time, and deep hypothermic circulatory arrest. Many clinical reports [6,7] suggest that drug dosage is the most important factor. However, the cutoff value for tranexamic acid dosage is unknown because of the variability among reports. Couture et al. reported that the number of convulsions with tranexamic acid administration was significantly higher in the high-dose group than in the low-dose group and was lower when the drug was administered over a longer period [5]. A meta-analysis by Gill et al. regarding the effect of tranexamic acid on total blood loss in spine surgery reported that the effect size of blood loss was −0.668 when tranexamic acid was used, indicating a significant decrease in blood loss [1]. In a previous CRASH study [2], tranexamic acid was shown to be useful in the treatment of trauma, and the number of deaths from blood loss after trauma was significantly lower in the tranexamic acid group (relative risk: 0.91, 95% confidence interval: 0.85–0.97). In orthopedic surgery, the efficacy of tranexamic acid in controlling bleeding in the perioperative period is well known, and the drug is now widely used. At present, the most common doses of tranexamic acid in orthopedic surgery are an initial intravenous infusion of 10 mg/kg, followed by a continuous intravenous infusion of 1–5 mg/kg/h, or pre-operative and post-operative intravenous infusions of 10–15 mg/kg or 1 g [15]. There are very few reports of serious side effects caused by tranexamic acid administration in the orthopedic field. A meta-analysis of 129 studies between 1972 and 2011 (10,488 patients) on thrombotic complications of tranexamic acid administration reported that tranexamic acid did not increase the risk of myocardial infarction, cerebral infarction, or venous thrombosis [15]. Therefore, orthopedic surgeons have high confidence in the safety of tranexamic acid. In the present case, seizures were observed after lumbar interbody fusion surgery and continued intermittently. Despite the patient’s low weight (49 kg), the total dose was a high volume of 3000 mg, and the blood concentration of 2000 mg immediately after the end of the surgery was further increased instantly by an intravenous injection. Tranexamic acid administration at a total dose of >80 mg/kg was considered as the cause of the seizures after laboratory tests ruled out other triggers. Tranexamic acid has a half-life of 1–1.5 h in the blood and is excreted renally within 3–4 h; however, the convulsions in our case continued until the 2nd post-operative day. To the best of our our knowledge, there have been no other reports of seizures caused by tranexamic acid administration in orthopedic surgery. Orthopedic surgeons should be aware of the serious side effects of tranexamic acid, as it is now frequently used in the perioperative period in orthopedic surgery.
This is the first report of a convulsive overload caused by the intravenous administration of tranexamic acid in the immediate period after orthopedic surgery. The seizures continued until the 2nd post-operative day. Fortunately, the seizures disappeared after intubation and administration of anticonvulsants, and the patient is now progressing without any sequelae. This adverse effect should be recognized when tranexamic acid is used, and caution should be exercised regarding the dosage.
To the best of our knowledge, this case is the first reported case of seizures after the administration of tranexamic acid during orthopedic surgery. Orthopedic surgeons and other involved clinicians need to recognize the possibility of this adverse effect and exercise caution when using tranexamic acid.
References
- 1.Gill JB, Chin Y, Levin A, Feng D. The use of antifibrinolytic agents in spine surgery. A meta-analysis. J Bone Joint Surg Am 2008;90:2399-407. [Google Scholar | PubMed]
- 2.CRASH-2 Trial Collaborators, Shakur H, Roberts I, Bautista R, Caballero J, Coats T, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): A randomized, placebo-controlled trial. Lancet 2010;376:23-32. [Google Scholar | PubMed]
- 3.Mohseni K, Jafari A, Nobahar MR, Arami A. Polymyoclonus seizure resulting from accidental injection of tranexamic acid in spinal anesthesia. Anesth Analg 2009;108:1984-6. [Google Scholar | PubMed]
- 4.Furtmüller R, Schlag MG, Berger M, Hopf R, Huck S, Sieghart W, et al. Tranexmic acid, a widely used antifibrinolytic agent, causes convulsions by a ɣ-aminobutyric acidA receptor antagonistic effect. J Phamacol Exp Ther 2002;301:168-73. [Google Scholar | PubMed]
- 5.Couture P, Lebon JS, Laliberté E, Desjardins G, Chamberland MÈ, Ayoub C, et al. Low-dose versus high-dose tranexamic acid reduces the risk of nonischemic seizures after cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2017;31:1611-7. [Google Scholar | PubMed]
- 6.Murkin JM, Falter F, Granton J, Young B, Burt C, Chu M. High-dose tranexamic acid is associated with nonischemic clinical seizures in cardiac surgical patients. Anesth Analg 2010;110:350-3. [Google Scholar | PubMed]
- 7.Myles PS, Smith JA, Forbes A, Silbert B, Jayarajah M, Painter T, et al. Tranexamic acid in patients undergoing coronary-artery surgery. N Engl J Med 2017;376:136-48. [Google Scholar | PubMed]
- 8.Manaka S, Ishijima B, Mayanagi Y. Postoperative seizures: Epidemiology, pathology, and prophylaxis. Neurol Med Chir (Tokyo) 2003;43:589-600. [Google Scholar | PubMed]
- 9.Arrowsmith JE, Grocott HP, Reves JG, Newman MF. Central nervous system complications of cardiac surgery. Br J Anaesth 2000;84:378-93. [Google Scholar | PubMed]
- 10.Burn J, Dennis M, Bamford J, Sandercock P, Wade D, Warlow C. Epileptic seizures after a first stroke: The oxfordshire community stroke project. BMJ 1997;315:1582-7. [Google Scholar | PubMed]
- 11.Pohlmann-Eden B, Newton M. First seizure: EEG and neuroimaging following an epileptic seizure. Epilepsia 2008;49:19-25. [Google Scholar | PubMed]
- 12.Fiebach JB, Schellinger PD, Jansen O, Meyer M, Wilde P, Bender J, et al. CT and diffusion-weighted MR imaging in randomized order: Diffusion-weighted imaging results in higher accuracy and lower interrater variability in the diagnosis of hyperacute ischemic stroke. Stroke 2002;33:2206-10. [Google Scholar | PubMed]
- 13.Fergusson DA, Hébert PC, Mazer CD, Fremes S, MacAdams C, Murkin JM, et al. A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med 2008;358:2319-31. [Google Scholar | PubMed]
- 14.Jiménez JJ, Iribarren JL, Brouard M, Hernández D, Palmero S, Jiménez A, et al. Safety and effectiveness of two treatment regimes with tranexamic acid to minimize inflammatory response in elective cardiopulmonary bypass patients: A randomized double-blind, dose-dependent, phase V clinical trial. J Cardiothorac Surg 2011;6:138. [Google Scholar | PubMed]
- 15.Ker K, Edwards P, Perel P, Shakur H, Roberts I. Effect of tranexamic acid on surgical bleeding: Systematic review and cumulative meta-analysis. BMJ 2012;344:e3054. [Google Scholar | PubMed]









