Early operative intervention in patients with diagnosed fluid collection and worsening neurological deficits after elective total hip arthroplasty can result in good functional outcomes.
Dr. Craig Goubeaux, Ohio Health, 5100 W. Broad St., Columbus, Ohio, 43228, USA. E-mail: goobs2442@gmail.com
Introduction: In this manuscript, we present a late presentation of deep peroneal nerve symptoms after total hip arthroplasty (THA) which subsequently completely resolved after seroma evacuation and sciatic nerve decompression. While hematoma formation causing deep peroneal nerve symptoms after THA has been reported in the literature, we are unaware of any reports of seroma formation causing similar symptoms.
Case Presentation: A 38-year-old female underwent an uncomplicated primary THA and developed paresthesia’s over the lateral leg and foot drop on post-operative day 7. Ultrasound diagnosed a fluid collection compressing the sciatic nerve. The patient underwent seroma evacuation and sciatic nerve decompression. Patient regained active dorsiflexion and minimal paresthesia’s over the dorsal lateral foot at the 12-month post-operative clinic visit.
Conclusion: Early operative intervention in patients with diagnosed fluid collection and worsening neurological deficits can result in good outcomes. This is a unique case as there are no other case reports of seroma formation causing deep peroneal nerve palsy.
Keywords: Seroma, deep peroneal nerve palsy, total hip arthroplasty, sciatic nerve, foot drop.
Sciatic nerve palsy is a well-documented and albeit rare complication after Total hip arthroplasty (THA) and accounts for 90% of all nerve injuries related to THA [1]. The incidence of sciatic nerve palsy after THA is approximately 1% in primary THA and up to 3%–8% in the revision setting with the highest in patients with developmental dysplasia of the hip (DDH) [1,2]. There have been several observed causes including tensioning or compression of the nerve due to retractor placement, manipulating the leg, during piriformis release, thermal injury, extravasation of cement, lengthening especially in DDH cases, and post-operative hematomas [3]. Post operative seroma formation after THA causing sciatic nerve palsy has not been well established within the literature. The peroneal nerve division of the sciatic nerve is more commonly injured than the tibial division due to its increased susceptibility to injury. One cause of this is the peroneal nerves’ relatively shorter distance between its points of fixation-the piriformis and fibular head-compared to the tibial nerve that travels from the piriformis to the tarsal tunnel [3]. Second, the tibial nerve has been shown to have a more robust epineurium, tightly wound perineum and overall, more fascicles compared to the peroneal and this theoretically affords the tibial nerve to withstand increased tension without sustaining significant damage [4]. Finally, the peroneal nerve is located laterally within the sciatic nerve thus placing it closer to the surgical field. Peroneal nerve palsy can be a devastating complication after THA which can lead to paresthesia’s and foot drop. This is a case of a young female patient who underwent an uncomplicated primary THA and developed paresthesia’s in the lateral leg and foot and eventually a foot drop. She was found to have compression of her sciatic nerve secondary to a fluid collection seen on ultrasound (US). The patient underwent seroma evacuation and sciatic nerve decompression 3 weeks after her primary THA. At her 12-month post-operative visit, she had almost complete resolution of symptoms.
A 38-year-old female presented to outpatient clinic with known advanced degenerative changes of the left hip and was having worsening pain and difficulty with ambulation. She failed other non-operative modalities and thus elected to undergo THA. She has normal body mass index. She has a past medical history of rheumatoid arthritis, scleroderma, gastroesophageal reflux disease, and tuberculosis. Her medications are Hydroxychloroquine (Plaquenil) 200 mg daily and Ferrous sulfate 325 mg. She does not take any anti-platelet or anti-coagulation medications. She has a history of right THA that had an uncomplicated post operative period. The patient underwent a THA using a standard posterior approach to the hip and had Stryker Trident 2 Acetabular component and Stryker Accolade 2 placed. There were no intraoperative complications. Post operative day (POD) 1, the patient was having appropriate amount of pain and denied any weakness, numbness, or tingling. Basic laboratories showed hemoglobin of 8.7 which is an appropriate drop from 10.7 preoperatively. She was able to work 30 min with physical and occupational therapy and ambulated 100 feet. She was discharged home POD1 with home healthcare. She was placed on Aspirin 325 mg twice daily. On POD 11, the patient called the office complaining of increased swelling over the lateral hip, and numbness and tingling from her knee down for the past 4 days. She followed up in the ED that day and was noted to have left sided foot drop and paresthesia’s in the L5 and S1 distribution. She then followed up in the office on POD 15 after musculoskeletal US and was found to have a fluid collection along the posterolateral hip and it appeared to be compressing the sciatic nerve. Plan was for evacuation of fluid collection with sciatic nerve decompression due to progressive neurological deterioration. The goal was to remove compression of the sciatic nerve from the fluid collection and any other tethers to aid in recovery of the sciatic nerve palsy. On incising the previous fascia lata closure a large, tense seroma was encountered and fully evacuated. No cultures taken as the seroma looked non infectious. Attention was then turned to the sciatic nerve. There were several adhesions surrounding the nerve causing secondary compression and limited excursion of the nerve (Fig. 1). Careful neurolysis with hemostats was performed proximally to the sciatic notch and distally to the gluteal sling. There was no evidence of tethering from previous posterior capsular repair. Manipulation of the hip through range of motion demonstrated overall improvement in excursion and mobility of the nerve (Fig. 2). Otherwise, hardware was intact, and no other causes of nerve compression were identified. A 10 French drain was placed deep to the fascia lata and removed POD1 with minimal output. The patient was discharged on POD 2 without significant improvement in paresthesia’s or foot drop. She followed up consistently and was given an AFO. At the 12 month post-operative visit, the patients’ paresthesias were nearly completely resolved over dorsolateral aspect of the foot and she was able to perform active and maintained dorsiflexion to 5–10 degrees past neutral.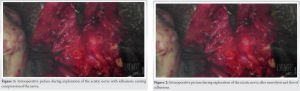
This is an interesting and unique case because this is a young, otherwise healthy patient that underwent primary THA and developed a seroma leading to a deep peroneal nerve palsy. The incidence of foot drop after primary THA has been reported to occur in 0.3–0.6% of cases [5]. The most common causes of sciatic nerve palsy are iatrogenic injury such as traction after lengthening, compression by retractors, manipulation of the leg, thermal injury, cement extrusion, hardware impingement, and post-operative subfascial hematoma [3]. Other risk factors that have been associated with motor nerve palsy after THA are pre-perative diagnosis of DDH or post-traumatic arthritis, use of a posterior approach, lengthening of the extremity and use of an uncemented femoral implant [6]. Understanding the many potential risk factors and causes of motor nerve palsy can help guide the surgeon to diagnosis and specific treatments. The first step in treating motor nerve palsy is diagnosis. Insult to the nerve begins intraoperatively. In one study, they found that 70% of patients after THA have subclinical sciatic nerve damage [7]. Patients with underlying peripheral neuropathy have an increased risk of post-operative palsy and this knowledge should influence the surgeon to take extra care intraoperatively with the use of force, retractors, and hardware placement to minimize injury to the sciatic nerve. Utilizing the posterior approach to the hip places the sciatic nerve, and specifically the peroneal division at risk due to manipulation of the leg, retractor placement, release, and repair of the piriformis. Dislocation of the hip through the posterior approach requires traction, hip flexion, and internal rotation, all of which can add increased strain to the sciatic nerve. Fleming et al. in their study of strain on the sciatic nerve during THA found a mean increase of 26% of strain on the nerve during hip flexion and knee extension [8]. Another area of possible injury is during piriformis release and repair. There exists in up to 20% of patient’s anatomic variants in the course of the sciatic nerve around the piriformis muscle [9]. Finally, it is retractor placement. In one cadaveric study, they found the sciatic nerve to be within 2 cm posteriorly to the acetabulum [10]. If care is taken intraoperatively, the sciatic nerve is protected throughout the case and the patient has no underlying medical conditions pre-disposing to neuropathy (e.g., diabetes mellitus, vitamin deficiencies, and thyroid dysfunction) then other causes must be explored. Investigation of other causes of neuropathy begins with imaging modalities. The most common and accessible modalities are US, X-ray, computed tomography scan, and magnetic resonance imaging. US is used when there is suspicion of post-operative hematoma or fluid collection as the first line imaging modality because it has high sensitivity, there is no metal artifact obscuring the image and no risk of radiation to the patient [11]. If a fluid collection is detected on US, the next step is forming a treatment plan. Deciding the between operative versus non-operative management of fluid collections is difficult. Studies have shown that operative intervention should be performed when the patient is having neurological deficits, concerns for infection, wound issues, or increasing pain [12,13,14]. One protocol in the literature for non-operative management of draining wounds secondary to fluid collections in total hips was to aspirate the fluid collection and send for cultures, close any open wounds, stop anticoagulants, reduce activity, and start antibiotics in select patients [15]. Whether operative or non-operative management is chosen, quick and decisive action must be taken to limit long-term complications. The patient presented here was having worsening neurological deficits with a fluid collection that was diagnosed using US after a primary total hip. This is a unique case as there are no other case reports of seroma formation causing deep peroneal nerve palsy. This case can aid in the decision-making process of when operative intervention should be pursued. Operative intervention was performed on POD 7 and this eventually lead to nearly complete resolution of symptoms at the 12-month office visit.
Early diagnosis and treatment of post-operative neurological deficits after primary THA are critical for good outcomes.
Hematoma formation has been reported in the literature but we report here the formation of a seroma causing deep peroneal nerve symptoms demonstrating the need to keep a broad differential when diagnosing and treating neurological symptoms after THA. This also reiterates using techniques to try and prevent seroma formation such as not undermining the soft tissues, tight fascial closure, possible drain placement in certain patient populations, etc.
References
- 1.Schmalzried TP, Amstutz HC, Dorey FJ. Nerve palsy associated with total hip replacement. Risk factors and prognosis. J Bone Joint Surg Am 1991;73:1074-80. [Google Scholar | PubMed]
- 2.DeHart MM, Riley LH Jr. Nerve injuries in total hip arthroplasty. J Am Acad Orthop Surg 1999;7:101-11. [Google Scholar | PubMed]
- 3.Su EP. Post-operative neuropathy after total hip arthroplasty. Bone Joint J 2017;99:46-9. [Google Scholar | PubMed]
- 4.Schraut NB, Walton S, Monsef JB, Shott S, Serici A, Soulii L, et al. What protects certain nerves from stretch injury? Anat Rec (Hoboken) 2016;299:111-7. [Google Scholar | PubMed]
- 5.O’Brien S, Gallagher N, Spence D, Bennett D, Dennison J, Beverland DE. Foot drop following primary total hip arthroplasty. Hip Int 2020;30:135-40. [Google Scholar | PubMed]
- 6.Farrell CM, Springer BD, Haidukewych GJ, Morrey BF. Motor nerve palsy following primary total hip arthroplasty. J Bone Joint Surg Am 2005;87:2619-25. [Google Scholar | PubMed]
- 7.Weale AE, Newman P, Ferguson IT, Bannister GC. Nerve injury after posterior and direct lateral approaches for hip replacement. A clinical and electrophysiological study. J Bone Joint Surg Br 1996;78:899-902. [Google Scholar | PubMed]
- 8.Fleming P, Lenehan B, O’Rourke S, McHugh P, Kaar K, McCabe JP. Strain on the human sciatic nerve in vivo during movement of the hip and knee. J Bone Joint Surg Br 2003;85:363-5. [Google Scholar | PubMed]
- 9.Pokorný D, Jahoda D, Veigl D, Pinskerová V, Sosna A. Topographic variations of the relationship of the sciatic nerve and the piriformis muscle and its relevance to palsy after total hip arthroplasty. Surg Radiol Anat 2006;28:88-91. [Google Scholar | PubMed]
- 10.Wang TI, Chen HY, Tsai CH, Hsu HC, Lin TL. Distances between bony landmarks and adjacent nerves: Anatomical factors that may influence retractor placement in total hip replacement surgery. J Orthop Surg Res 2016;11:31. [Google Scholar | PubMed]
- 11.Nishii T, Sakai T, Takao M, Yoshikawa H, Sugano N. Is ultrasound screening reliable for adverse local tissue reaction after hip arthroplasty? J Arthroplasty 2014;29:2239-44. [Google Scholar | PubMed]
- 12.Yuan HF, Yang MY, Xu WD. Late sciatic nerve palsy caused by recurrent hematoma after primary total hip arthroplasty: A case report. Orthop Surg 2013;5:222-4. [Google Scholar | PubMed]
- 13.Butt AJ, McCarthy T, Kelly IP, Glynn T, McCoy G. Sciatic nerve palsy secondary to postoperative haematoma in primary total hip replacement. J Bone Joint Surg Br 2005;87:1465-7. [Google Scholar | PubMed]
- 14.Wagenaar FB, Löwik CA, Zahar A, Jutte PC, Gehrke T, Parvizi J. Persistent wound drainage after total joint arthroplasty: A narrative review. J Arthroplasty 2019;34:175-82. [Google Scholar | PubMed]
- 15.Reich MS, Ezzet KA. A nonsurgical protocol for management of postarthroplasty wound drainage. Arthroplast Today 2017;4:71-3. [Google Scholar | PubMed]








