The two-step procedure of open biopsy followed by ethanol injection using the open approach for a venous hemangioma of spine allowed accurate diagnosis and effective treatment.
Dr. Juichi Tonosu, Department of Orthopedic Surgery, Kanto Rosai Hospital, Kanagawa, Japan. E-mail: juichitohnosu@yahoo.co.jp
Introduction: Venous hemangiomas of the thoracic spine are rare tumors that are diagnose based on radiological findings. Ethanol sclerosis therapy through the percutaneous or open approaches has been reported to be useful treatment options. Therefore, radiological examination and the treatment procedure can be performed together. As pathological diagnosis of the tumor is important, a strategy that comprises biopsy followed by definitive treatment is ideal. The tips and complications of the two-step open procedure for ethanol sclerosis therapy have not been discussed in detail. This is the first report of this kind in the literature, especially about the tips and complications.
Case Report: A 51-year-old woman presented with pain in the upper part of her back. Radiological examination revealed a hypervascular tumor at the second thoracic vertebra. We first performed an open biopsy along with decompression and fixation surgery, because the patient developed a walking disability with motor weakness in her right leg. The tumor was pathologically diagnosed as a venous hemangioma. Therefore, we performed ethanol sclerosis therapy using the open approach as a curative technique for the tumor 17 days after the initial surgery. A total of 10 mL of a mixture of 100% ethanol and a lipid-soluble contrast medium - which improve visibility - was injected intermittently and slowly. This was followed by the injection of 3 mL of a water-soluble contrast medium to confirm sclerosis. Immediately after the last procedure, the amplitudes of motor-evoked potentials in all bilateral lower extremity muscles disappeared simultaneously. The patient incomplete paralysis of the lower extremity and transient dysuria postoperatively; however, she could walk without assistance after 5 months.
Conclusion: This case highlights the following: First, the two-step procedure of open biopsy followed by ethanol injection using the open approach allowed accurate diagnosis and effective treatment. Second, additional injection of a water-soluble contrast medium to confirm sclerosis after ethanol injection can cause paralysis. Third, a mixture of ethanol and a lipid-soluble contrast medium effective improves visibility to identify expansions. These experiences will be useful for following ethanol sclerosis therapy for a venous hemangioma of the thoracic spine.
Keywords: Venous hemangioma, thoracic spine, ethanol sclerosis therapy, two-step open procedure, paralysis, additional injection, water-soluble contrast medium, visibility, lipid-soluble contrast medium, tips, complication.
Venous hemangiomas of the thoracic spine are rare, benign tumors. They are often found incidentally in radiological examinations and have few symptoms; however, some venous hemangiomas are symptomatic and gradually increase in size [1,2]. Various therapeutic approaches have been proposed and used to manage hemangiomas of the spine [3], including vertebroplasty using cement [4], radiation therapy [5], transarterial embolization [6], and complete tumor resection [7,8]. Ethanol sclerosis therapy was first reported by Heiss et al. in 1994 [9]. In this technique, highly concentrated ethanol is injected into the hemangioma to induce coagulative necrosis of the vascular endothelium and surrounding tissues, which is followed by irreversible vascular occlusion [10]. This technique has been reported to show good results [11,12,13], although some case reports have described complications, such as paralysis [12] and delayed vertebral compression fractures [13]. All of the reported therapies were not performed on the basis of pathological diagnoses but based on radiological diagnoses, and several reported procedures were performed percutaneously under computed tomography (CT) guidance. We report our findings in the case of a patient who was diagnosed through open biopsy, received ethanol sclerosis therapy in a two-step open procedure, and showed incomplete paralysis of the lower extremity and transient dysuria.
A 51-year-old woman with no relevant medical history presented with pain in the upper part of her back for 2 weeks. There was no neurological deficit in either the trunk or the lower extremities at her first visit. A plain radiograph showed a radiolucent zone in the spinous process of the second thoracic spine (T2). CT revealed osteolytic changes not only in the spinous process but also in the vertebral body, pedicles, and lamina of T2, which expanded into the spinal canal. The changes were not accompanied by cortical destruction (Fig. 1). 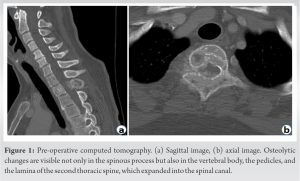 Magnetic resonance imaging (MRI) revealed an area with low intensity on T1-weighted imaging (T1WI) and high intensity on T2-weighted imaging (T2WI) and short inversion time inversion recovery imaging that compressed the spinal cord from the posterior and the right sides, which was assumed to be a hypervascular tumor. The area had an extraosseous region on the right (Fig. 2).
Magnetic resonance imaging (MRI) revealed an area with low intensity on T1-weighted imaging (T1WI) and high intensity on T2-weighted imaging (T2WI) and short inversion time inversion recovery imaging that compressed the spinal cord from the posterior and the right sides, which was assumed to be a hypervascular tumor. The area had an extraosseous region on the right (Fig. 2). 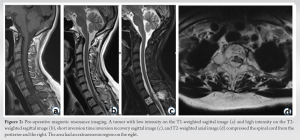 Blood tests did not reveal any abnormal findings. A CT-guided percutaneous biopsy did not yield a histological diagnosis. Over the 2-week course of these examinations, the patient developed a walking disability with motor weakness in her right leg. Therefore, we performed an open biopsy along with T2 laminectomy, and posterolateral fusion from C7 to T4 levels (Fig. 3).
Blood tests did not reveal any abnormal findings. A CT-guided percutaneous biopsy did not yield a histological diagnosis. Over the 2-week course of these examinations, the patient developed a walking disability with motor weakness in her right leg. Therefore, we performed an open biopsy along with T2 laminectomy, and posterolateral fusion from C7 to T4 levels (Fig. 3). Histological examination revealed that the tumor was a venous hemangioma. We adopted the second surgery for ethanol sclerosis therapy with an open approach as a curative technique for the tumor at 17 days after the initial surgery. Transcranial motor-evoked potential (MEP) monitoring the lower extremities was applied throughout the surgery to evaluate spinal cord function. The same skin incision was made as in the initial surgery. A 14-gauge cannulated needle for biopsy was inserted through the left pedicle. Before the ethanol injection, 3 mL of a water-soluble contrast medium (Omnipaque240®) was injected into the vertebral body through the needle, and the absence of leakage into the epidural space was confirmed. The contrast disappeared within 2 min, which indicated that the tumor was hypervascular. Next, a mixture of 7.5 mL of 100% ethanol and 2.5 mL of a lipid-soluble contrast medium (Lipiodol®) was used to visibly identify the expansion of ethanol, that is, 75% of ethanol was prepared in a 10 mL syringe with a lock. The dura mater was covered with thick gauze to protect it from drops of ethanol. Ethanol was injected intermittently: a series of ethanol injections (0.5-mL ethanol injection followed by 2 min of rest) was administered carefully with fluoroscopy and MEP monitoring until a total volume of 10 mL was administered. At the end of the injection, the contrast agent filled the vertebral body and extraosseous region without epidural infiltration (Fig. 4), and the amplitudes of MEPs in the lower extremities did not decrease. Next, 3 mL of a water-soluble contrast medium was injected again 2 min after the final 0.5-mL ethanol injection to identify sclerosis of the vertebral body. The contrast did not disappear for over 3 min, indicating that the sclerosis was complete. However, the amplitudes of MEPs in all bilateral lower extremities disappeared simultaneously. The patient presented with incomplete paralysis in the right leg immediately after extubation. The manual muscle testing (MMT) scores of the iliopsoas, quadriceps, tibialis anterior, and gastrocnemius on the right were 2/5, 2/5, 0/5, and 2/5, respectively. Post-operative CT showed no leakage of the mixture of ethanol and the lipid-soluble contrast medium into the spinal canal. MRI performed immediately after the surgery showed no spinal cord compression and no intensity change in the spinal cord. MRI also showed intensity changes of the tumor, which now showed high intensity on T1WI and low intensity on T2WI, indicating completion of sclerosis (Fig. 5a, b, c). The patient’s paralysis gradually improved, and the MMT scores of the muscles were 4–5 in 4 weeks. However, a persistent abnormality of sensation, especially in proprioception of the right leg, resulted in delayed recovery of the patient’s walking ability. She could walk with a walker at 6 weeks and without any assistance at 5-month postoperatively. Moreover, while she was unable to urinate herself or feel the urge to urinate after surgery, dysuria improved at 2 weeks and fully recovered at 4 weeks after surgery. Post-operative MRI at 2 weeks after surgery revealed expansion of the high-intensity area on T2WI in the spinal cord (Fig. 5d, e), which reduced and was limited to the posterior funiculus as time passed. The area remained in the post-operative MRI performed 6 months after surgery (Fig. 5f, g).
Histological examination revealed that the tumor was a venous hemangioma. We adopted the second surgery for ethanol sclerosis therapy with an open approach as a curative technique for the tumor at 17 days after the initial surgery. Transcranial motor-evoked potential (MEP) monitoring the lower extremities was applied throughout the surgery to evaluate spinal cord function. The same skin incision was made as in the initial surgery. A 14-gauge cannulated needle for biopsy was inserted through the left pedicle. Before the ethanol injection, 3 mL of a water-soluble contrast medium (Omnipaque240®) was injected into the vertebral body through the needle, and the absence of leakage into the epidural space was confirmed. The contrast disappeared within 2 min, which indicated that the tumor was hypervascular. Next, a mixture of 7.5 mL of 100% ethanol and 2.5 mL of a lipid-soluble contrast medium (Lipiodol®) was used to visibly identify the expansion of ethanol, that is, 75% of ethanol was prepared in a 10 mL syringe with a lock. The dura mater was covered with thick gauze to protect it from drops of ethanol. Ethanol was injected intermittently: a series of ethanol injections (0.5-mL ethanol injection followed by 2 min of rest) was administered carefully with fluoroscopy and MEP monitoring until a total volume of 10 mL was administered. At the end of the injection, the contrast agent filled the vertebral body and extraosseous region without epidural infiltration (Fig. 4), and the amplitudes of MEPs in the lower extremities did not decrease. Next, 3 mL of a water-soluble contrast medium was injected again 2 min after the final 0.5-mL ethanol injection to identify sclerosis of the vertebral body. The contrast did not disappear for over 3 min, indicating that the sclerosis was complete. However, the amplitudes of MEPs in all bilateral lower extremities disappeared simultaneously. The patient presented with incomplete paralysis in the right leg immediately after extubation. The manual muscle testing (MMT) scores of the iliopsoas, quadriceps, tibialis anterior, and gastrocnemius on the right were 2/5, 2/5, 0/5, and 2/5, respectively. Post-operative CT showed no leakage of the mixture of ethanol and the lipid-soluble contrast medium into the spinal canal. MRI performed immediately after the surgery showed no spinal cord compression and no intensity change in the spinal cord. MRI also showed intensity changes of the tumor, which now showed high intensity on T1WI and low intensity on T2WI, indicating completion of sclerosis (Fig. 5a, b, c). The patient’s paralysis gradually improved, and the MMT scores of the muscles were 4–5 in 4 weeks. However, a persistent abnormality of sensation, especially in proprioception of the right leg, resulted in delayed recovery of the patient’s walking ability. She could walk with a walker at 6 weeks and without any assistance at 5-month postoperatively. Moreover, while she was unable to urinate herself or feel the urge to urinate after surgery, dysuria improved at 2 weeks and fully recovered at 4 weeks after surgery. Post-operative MRI at 2 weeks after surgery revealed expansion of the high-intensity area on T2WI in the spinal cord (Fig. 5d, e), which reduced and was limited to the posterior funiculus as time passed. The area remained in the post-operative MRI performed 6 months after surgery (Fig. 5f, g).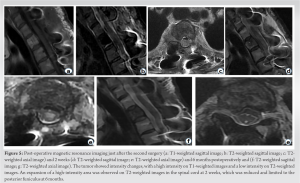
Ethanol sclerosis therapy has been used for the treatment of venous hemangiomas of the thoracic spine [11,12,13]. Ethanol is effective against tumors but is harmful to normal tissues; therefore, we regarded a careful definite diagnosis as essential for the appropriate application of ethanol sclerosis therapy. After the failure of histological examination through a percutaneous biopsy, we performed an open biopsy along with decompression and fixation surgery. The results provided a histological diagnosis of a venous hemangioma. At that point, careful observation without any additional surgery was an option because the preoperative motor weakness recovered to full strength, and also because a venous hemangioma is a benign tumor. However, we adopted a curative technique for the tumor because the total resection of the enlarged tumor at the T2 level in the future seemed to be technically difficult to complete even if the tumor was slow-growing. Ethanol sclerosis therapy was then performed via the open approach. One advantage of the open procedure compared to the CT-guided procedure was that appropriate placement of the needle was easy, while a disadvantage was the potential for ethanol dripping on the dura mater directly during the injection. We covered the dura mater with thick gauze and used a syringe with a lock to avoid accidental dripping of ethanol., which was an important consideration. Although a direct approach through the open procedure has been reported [11], the two-step open procedure for ethanol sclerosis therapy has not been described previously, and it can be recommended to avoid misdiagnosis. Post-operative incomplete paralysis occurred immediately after surgery in our patient. In one case series, one out of 33 cases showed worsened paralysis after the injection [11]. Another case series demonstrated transient neurologic deterioration and subsequent recovery in all 14 cases; however, the causes were not discussed [13]. The timing of the disappearance of MEP amplitudes could indicate that the final injection of water-soluble contrast medium may have been responsible for the paralysis. The total amount of ethanol and contrast medium may also be associated with the occurrence of paralysis. After the initial water-soluble contrast medium had disappeared, a total volume of 13 mL, consisting of 10 mL of 75% ethanol and 3 mL of water-soluble contrast medium, was injected into the vertebral body. The mean amount of injected ethanol in some case series has been reported to be approximately 10–15 mL [11,12,13]. The volume of the T2 vertebral body in our case could be smaller than those of previously reported thoracolumbar cases. On the other hand, the injection speed could be responsible for paralysis. Although ethanol was intermittently injected in small amounts, the subsequent final injection of water-soluble contrast medium was administered at once. Sclerosis of the vessels caused by the final injection of ethanol may not have been completed when the water-soluble contrast medium was injected. There were some possible hypotheses for the paralysis. First, it could be direct extrusion of residual ethanol to the spinal canal through the fragile posterior wall of the vertebral body; however, there were no injuries to the anterior part of the spinal cord on postoperative MRI immediately after the surgery. In addition, the residual high-intensity area on T2WI at both 6 weeks and 6 months after the surgery was limited to the posterior cord. These findings did not support the hypothesis of direct extrusion. Second, ischemia of the spinal cord may have been caused by the rapid sclerosis of feeding arteries in the spinal cord, in addition to the hypervascular vertebral body. Endovascular embolization for vertebral hemangioma has been reported to cause incomplete paralysis, and the mechanism was assumed to be spinal cord ischemia [14]. The clinical and radiological findings in that case were similar to those in our case. The initial report of ethanol injection therapy recommended slow injection of 2 mL/ 5–10 min [9]. Although the report did not provide a reason for this, it may have been to avoid rapid changes in vessel flow around the spinal cord. Third, compensatory reperfusion injury of the spinal cord next to the sclerosed region have could occurred. The initial report also recommended injection of a water-soluble contrast medium before and after ethanol injection [9]. The pre-injection was intended to ensure no leakage into the epidural space and rapid disappearance of the contrast medium caused by rich vessel flow, whereas the after-injection was intended to ensure slow disappearance of the contrast medium with the completion of sclerosis. In addition to this method, we adopted the method of using a mixture of ethanol and a lipid-soluble contrast medium, which has not been reported for vertebral hemangioma but for renal carcinoma [15]. This method was effective in identifying the expansion of ethanol visibly during the procedure. The lipid-soluble contrast medium did not disappear postoperatively; therefore, a post-operative CT could demonstrate appropriate expansion of ethanol. These findings suggest that the after-injection of water-soluble contrast medium for confirmation, which could have caused the paralysis, may not be necessary. A limitation of this approach was that the MEP findings could not completely and simultaneously indicate the occurrence of paralysis.
We experienced the two-step procedure of open biopsy followed by ethanol injection using the open approach for a venous hemangioma of the thoracic spine, which facilitated accurate diagnosis and effective therapy. The cause of post-operative incomplete paralysis and the tip of identifying the expansion of ethanol visibly during the procedure were discussed in detail.
First, the two-step procedure allowed accurate diagnosis and effective treatment. Second, additional injection of a water-soluble contrast medium to confirm sclerosis after ethanol injection can cause paralysis. Third, a mixture of ethanol and a lipid-soluble contrast medium effective improves visibility to identify expansions. These experiences will be useful for following ethanol sclerosis therapy for a venous hemangioma of the thoracic spine.
References
- 1.Laredo JD, Reizine D, Bard M, Merland JJ. Vertebral hemangiomas: Radiologic evaluation. Radiology 1986;161:183-9. [Google Scholar | PubMed]
- 2.Gaudino S, Martucci M, Colantonio R, Lozupone E, Visconti E, Leone A, et al. A systematic approach to vertebral hemangioma. Skeletal Radiol 2015;44:25-36. [Google Scholar | PubMed]
- 3.Cloran FJ, Pukenas BA, Loevner LA, Aquino C, Schuster J, Mohan S. Aggressive spinal haemangiomas: Imaging correlates to clinical presentation with analysis of treatment algorithm and clinical outcomes. Br J Radiol 2015;88:20140771. [Google Scholar | PubMed]
- 4.Nambiar M, Maingard JT, Onggo JR, Phan K, Asadi H, Brooks DM, et al. Single Level percutaneous vertebroplasty for vertebral hemangiomata – A review of outcomes. Pain Physician 2020;23:E637-42. [Google Scholar | PubMed]
- 5.Parekh AD, Amdur RJ, Mendenhall WM, Morris CG, Zlotecki RA. Long-term tumor control with radiotherapy for symptomatic hemangioma of a vertebral body. Spine (Phila Pa 1976) 2019;44:E731-4. [Google Scholar | PubMed]
- 6.Smith TP, Koci T, Mehringer CM, Tsai FY, Fraser KW, Dowd CF, et al. Transarterial embolization of vertebral hemangioma. J Vasc Interv Radiol 1993;4:681-5. [Google Scholar | PubMed]
- 7.Ji X, Wang S, Oner FC, Bird JE, Lu N. Surgical management of enneking stage 3 aggressive vertebral hemangiomas with neurological deficit by one-stage posterior total en bloc spondylectomy: A review of 23 cases. Spine (Phila Pa 1976) 2020;45:E67-75. [Google Scholar | PubMed]
- 8.Acosta FL Jr., Sanai N, Cloyd J, Deviren V, Chou D, Ames CP. Treatment of Enneking stage 3 aggressive vertebral hemangiomas with intralesional spondylectomy: Report of 10 cases and review of the literature. J Spinal Disord Tech 2011;24:268-75. [Google Scholar | PubMed]
- 9.Heiss JD, Doppman JL, Oldfield EH. Brief report: Relief of spinal cord compression from vertebral hemangioma by intralesional injection of absolute ethanol. N Engl J Med 1994;331:508-11. [Google Scholar | PubMed]
- 10.Ellman BA, Parkhill BJ, Marcus PB, Curry TS, Peters PC. Renal ablation with absolute ethanol. Mechanism of action. Invest Radiol 1984;19:416-23. [Google Scholar | PubMed]
- 11.Chandra SP, Singh P, Kumar R, Agarwal D, Tandon V, Kale SS, et al. Long-term outcome of treatment of vertebral body hemangiomas with direct ethanol injection and short-segment stabilization. Spine J 2019;19:131-43. [Google Scholar | PubMed]
- 12.Bas T, Aparisi F, Bas JL. Efficacy and safety of ethanol injections in 18 cases of vertebral hemangioma: A mean follow-up of 2 years. Spine (Phila Pa 1976) 2001;26:1577-82. [Google Scholar | PubMed]
- 13.Goyal M, Mishra NK, Sharma A, Gaikwad SB, Mohanty BK, Sharma S. Alcohol ablation of symptomatic vertebral hemangiomas. AJNR Am J Neuroradiol 1999;20:1091-6. [Google Scholar | PubMed]
- 14.Fernandez-Torron R, Palma JA, Riverol M, Irimia P, Martinez-Vila E. Brown-sequard syndrome after endovascular embolization of vertebral hemangioma. Spinal Cord 2012;50:636-7. [Google Scholar | PubMed]
- 15.Park JH, Jeon SC, Kang HS, Im JG, Han MC, Kim CW. Transcatheter renal arterial embolization with the mixture of ethanol and iodized oil (Lipiodol). Invest Radiol 1986;21:577-80. [Google Scholar | PubMed]