Direct anterior approach with an extension table is a promising advancement in primary total hip arthroplasty that has the potential to improve patient outcomes and reduce surgical complications in a safe and refined manner.
Dr. Supreet Bajwa, Consultant, Wockhardt Hospitals, Mumbai, Maharashtra, India. E-mail: info.bajwasorthopaedic@gmail.com
Introduction: Through the use of a natural intramuscular and internervous interval, the direct anterior approach (DAA) for total hip arthroplasty (THA) has been suggested to have several advantages over other popular arthroplasty approaches. The usage of DAA for THA has considerably grown in the West in recent years due to the emphasis on tissue preservation and minimally invasive joint replacements. However, due to the long learning curve, literature on this approach originating from India has been inadequate, suggesting a limited adoption of this surgical technique by the Indian diaspora of practicing surgeons.
Technical Description: The DAA for THA is reliable and suitable for all conventional primary and revision THA cases. In the surgical procedure, the patient is positioned supine on the standard operating room (OR) table with the legs positioned in the Leg Positioning Traction System. The OR table may rotate, which enables the surgeon to perform the surgery more conveniently as it covers the majority of the patient’s center of gravity. The incision is made over the tensor fascia lata. After femoral neck osteotomy, the head is removed, and traction is applied to the operative leg followed by acetabular cup insertion. The femoral stem is inserted after releasing soft tissues around the proximal femur using the leg positioning traction system. Using traction, flexion, and internal rotation, the femoral head is pushed into the acetabulum at the same time, and reduction is achieved.
Conclusion: DAA for THA technique offers patients the advantage of minimally invasive surgery compared to other approaches. Many authors have published their experiences and technical keys to successfully completing this procedure, and several variations of the procedure have been described. The approach described is implemented utilizing specifically developed instruments, including a specialized table and intraoperative fluoroscopy while employing the standard surgical incision. This article attempts to outline the authors’ technique for performing the DAA in the supine position for a primary THA using a Leg Positioning Traction System, with a focus on technical details in assisting an early DAA convert in making a safe transition.
Keywords: Direct anterior approach, surgical technique, total hip arthroplasty, traction table.
The direct anterior approach (DAA) for total hip arthroplasty (THA) was first described in 1985 by the Judet brothers using an orthopedic traction table [1]. Emile Letournel, one of Judet’s residents, continued to use the table for pelvic and acetabular fracture surgeries. Letournel had two trainees, Frederic Laude and Joel Matta, who eventually contributed to the advancement of the orthopedic table in modern arthroplasty. In the early 1990s, Laude developed the technique in Paris on a table he built in his garage. The orthopedic table was first used in the United States by Matta et al [2]. Over the past decade, DAA for THA has emerged as a pivotal technique with profound implications. Departing from conventional surgical methods, the DAA has garnered substantial attention due to its potential to optimize patient outcomes [3] expedite post-operative recovery [4], an improve post-operative hip stability profile [5,6,7], and potentially less muscle damage [8,9]. Comparisons of DAA with or without a traction table produce indefinite and inconclusive results, but there appears to be no difference in the rate of complications, such as an intraoperative femur fracture, between the two techniques [10]. However, DAA for THA is a technically challenging operation with a well-defined learning curve [11,12,13] and several potential complications, including femur fractures [14], perforations [15], and lateral femoral cutaneous nerve (LFCN) injury [16]. Furthermore, it can be difficult to sufficiently expose the acetabulum and femur without causing excessive tension on surrounding tissues [17]. Compounding these known challenges with our patients’ delayed presentation and aversion to surgery, DAA is a surgical technique that should be approached with caution to maintain its predictable outcomes in Indian population. Here, we describe DAA as an approach for primary THA that uses a leg position traction system (LPTS) [Purist, IOT Orthopaedics, Switzerland] as an adjunct to overcome the challenges of cases in a more consistent, reproducible, and efficient manner while also being mobile and compact to fit in any theatre setup across India.
The DAA has been promoted by several authors for usage in almost all body habitus and hip disorders [18]. The ideal patient has been described as flexible, and non-muscular, with a valgus femoral neck and a favorable femoral offset [19]. It is rational to begin by developing the ability with thinner patients with a body mass index ≤25 kg/m2. Although appropriate exposure to the hip is possible through the DAA technique, it has also been recognized that a lack of instrumentation (Fig. 1) designed for DAA is a contraindication for comfortable early adoption.  Certain anatomical characteristics of the native hip and pelvis make the DAA more challenging: (i) a broad or horizontal iliac wing restricts access to the femoral canal during broaching and femoral component implantation, (ii) acetabular protrusions bring the femoral canal closer to the pelvis, obstructing the femoral access, and (iii) a neck shaft angle with a reduced offset position in the femoral canal deeper in the thigh. Besides, the anatomy associated with obese muscular males limits the available space for component placement. One disadvantage of the DAA is that access to the posterior column of the pelvis is limited. Anterior exposure might not be acceptable if the patient has a weak posterior acetabular wall as a result of prior hardware or trauma, or if posterior acetabular augmentation is intended [18].
Certain anatomical characteristics of the native hip and pelvis make the DAA more challenging: (i) a broad or horizontal iliac wing restricts access to the femoral canal during broaching and femoral component implantation, (ii) acetabular protrusions bring the femoral canal closer to the pelvis, obstructing the femoral access, and (iii) a neck shaft angle with a reduced offset position in the femoral canal deeper in the thigh. Besides, the anatomy associated with obese muscular males limits the available space for component placement. One disadvantage of the DAA is that access to the posterior column of the pelvis is limited. Anterior exposure might not be acceptable if the patient has a weak posterior acetabular wall as a result of prior hardware or trauma, or if posterior acetabular augmentation is intended [18].
Setup and Positioning
The patient lies in a supine position on a standard OR table. After the desired anesthesia, the perineal operative post is fixed to the OR side rail of the operative side. The author advocates the use of general anesthesia in the initial learning curve as the time taken to complete the procedure may be longer than the standard duration of spinal anesthesia. General anesthesia also helps the muscle relax more than spinal anesthesia which may help in the access to the femur for its preparation. Attached to the OR table’s side rail, the opposite leg holder (OLH) holds the non-operative leg in position (Fig. 2a and b). In general, the OR table is rotated so that the long portion of the table base is under the patient’s center of gravity, allowing for adequate C-Arm maneuverability. The patient’s legs are wrapped in a soft roll, and both traction boots are secured. The patient is then pulled to the perineal post and the operative side boot is attached to the LPTS following which the contralateral limb is attached to the OLH. In the rare case of surgical incision extension, the operative side is prepared and draped sterilely from the umbilicus to the patella, while the anterior superior iliac spine (ASIS) remains within the operative field (Fig. 3). 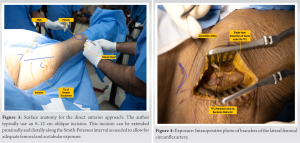 With obese patients, the pannus should be retracted with adhesive tape to prevent any potential interference with the exposure. Through the incision, the ASIS is kept intact as a surgical landmark.
With obese patients, the pannus should be retracted with adhesive tape to prevent any potential interference with the exposure. Through the incision, the ASIS is kept intact as a surgical landmark.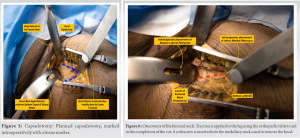
Surgical Approach
The incision commences 1 cm distally and 2 cm laterally from the ASIS and extends between 8 and 12 cm. The incision is slightly oblique and directed at the tip of the fibula. Before making the surgical incision, the author suggests palpating the tensor fascia lata (TFL) muscle and centering the surgical incision over the belly of the TFL. After dissecting the subcutaneous tissue, score the fascia of the TFL and mobilize the TFL laterally. A well-known complication of this method is the proximity of the incision to the LFCN. Although it is commonly assumed that staying lateral to the TFL/Sartorius interval lowers the risk of nerve injury, a cadaveric study of LFCN arborization revealed that the gluteal branch crossed the anterior margin of the TFL at 44 mm from the ASIS, and the femoral branch crossed this margin in half of the specimens, at an average of 46 mm distal to the ASIS [20]. Once this interval (Heuter interval) is established between the TFL (laterally) and the Rectus Femoris (medially), the fascia is gently split to expose the approach’s working window. The branches of the lateral circumflex femoral artery (Fig. 4) are identified and cauterized just beneath the fascia using electrocautery. These vessels generally lie at the level of the Greater Trochanter and should bisect the surgical incision which can be marked using fluoroscopy guidance at the planning of the surgical incision. The pericapsular fat is removed, and two extraarticular blunt Hohmann retractors are inserted: one inferomedially over the medial side of the neck, proximal to the Lesser Trochanter, and the other superolaterally between the capsule and the Gluteus Medius muscle belly. 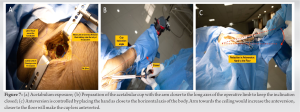 After elevating the indirect head of Rectus Femoris on the anterior column of the acetabulum, a third sharp retractor is placed under the reflected head origin at the superior aspect of the acetabulum. At this point, the capsulotomy is marked (the author prefers an inverted V-shaped capsulotomy) and the flap is tagged with a non-absorbable suture (Fig. 5). Other methods of performing the capsulotomy are an inverted T-shaped or H-shaped capsulotomy. If working space is limited, the author does not hesitate to perform a capsulectomy at this stage. All three retractors are then repositioned intracapsularly, and a femoral neck osteotomy is performed (Fig. 6).
After elevating the indirect head of Rectus Femoris on the anterior column of the acetabulum, a third sharp retractor is placed under the reflected head origin at the superior aspect of the acetabulum. At this point, the capsulotomy is marked (the author prefers an inverted V-shaped capsulotomy) and the flap is tagged with a non-absorbable suture (Fig. 5). Other methods of performing the capsulotomy are an inverted T-shaped or H-shaped capsulotomy. If working space is limited, the author does not hesitate to perform a capsulectomy at this stage. All three retractors are then repositioned intracapsularly, and a femoral neck osteotomy is performed (Fig. 6).  Traction is applied to the traction table for about 2–3 cm to create space within the joint for the femoral head to be extracted with a corkscrew T-handle extractor. In cases with coxa vara, this extraction process may be difficult due to the lack of space to extract the femoral head. In such cases, a double-level wedge osteotomy of the femoral neck can be performed to lever out the head easily.
Traction is applied to the traction table for about 2–3 cm to create space within the joint for the femoral head to be extracted with a corkscrew T-handle extractor. In cases with coxa vara, this extraction process may be difficult due to the lack of space to extract the femoral head. In such cases, a double-level wedge osteotomy of the femoral neck can be performed to lever out the head easily.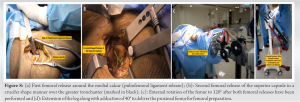
Acetabular Preparation and Component Placement
Following head extraction, the author prefers to remove all retractors and insert a fixed-angle U-retractor to expose the acetabulum. For a left hip DAA approach in patients with a bulky rectus, acetabular retractors are placed anterosuperior, anteroinferiorly, and posteriorly at 10,’ 6,’ and 4’o clock, respectively. If the labrum is ossified, it is removed with electrocautery or an osteotome to optimize acetabular reamer placement. To medialize the cup to the level of the teardrop symbolizing the acetabulum floor, the initial reamer is positioned at 90° or perpendicular to the body’s long axis. Once sufficiently medialized, larger reamers are introduced sequentially under intraoperative fluoroscopy (IOF) guidance at the desired inclination and anteversion (Fig. 7a, b, c). As a rule of thumb, the author prefers 40°–43° of targeted inclination and about 15° of anteversion. The final acetabular component is placed under IOF C-Arm guidance after the bony bed has been cleaned with irrigation. The final component is installed, including the acetabular liner, and attention is turned to the femoral side.
As a rule of thumb, the author prefers 40°–43° of targeted inclination and about 15° of anteversion. The final acetabular component is placed under IOF C-Arm guidance after the bony bed has been cleaned with irrigation. The final component is installed, including the acetabular liner, and attention is turned to the femoral side.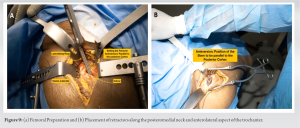
Femoral Preparation and Component Placement
This is the most important stage of DAA in the learning phase of implementing the procedure, as lack of femoral exposure may lead to complications in the early learning of this procedure. The LPTS consistently improves proximal femoral access and delivery, which is critical to avoid known complications of femoral preparations such as canal perforation, calcar fractures, and greater trochanteric fractures [21]. For this stage of surgery, the author prefers to use LPTS. First, the femur is manually rotated externally to the most easily achievable position with reasonable resistance. As LPTS handling (non-scrubbed) assistant follows the primary surgeon, the external rotation will be limited in contracted hips with long-standing deformities. In contrast, greater rotations can be attained in supple hips or patients with neck of femur fractures (Fig. 8a, b, c, d). After that, the first femoral release is performed by electrocauterizing the capsule around the calcar to the lesser trochanter, this release aids in further femoral rotation. The highest rotation that can be achieved with this LPTS is 140°, which, according to the authors’ previous research and experience, is adequate even for the most severe hip deformities. Before moving on to the subsequent release of the superior capsule, the author places a Hohmann retractor around the calcar at the posteromedial region of the femoral neck. This is done before moving on to the subsequent release of the superior capsule [22], debulking it to visualize the tip of the greater trochanter. Even in the most deformed hips, no further release is usually required after this step. Limiting this superior capsular release is thought to improve hip joint stability. To avoid damaging the Conjoint tendon, this release is extended but not beyond the piriformis fossa. With these releases and retractors in place, the exposed femoral neck is ready to be delivered into the field of view by extending the leg on the LPTS. The LPTS has a maximum extension of 90°. Adducting the leg on the LPTS by 40°, the visibility of the femoral neck surface is improved significantly. The author suggests releasing the Conjoint tendon if better visualization is required, but not as a routine step in femoral exposure. The piriformis tendon can also be released to increase exposure, but care must be taken to avoid releasing the Obturator Externus tendon, which is an important dynamic hip stabilizer. To avoid perforation during broaching, the author uses a starting rasp to sound the femoral canal (Fig. 9a and b).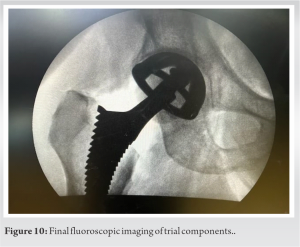 Broaching begins by gradually increasing the size of the broaches until an adequate fit from the medial to the lateral cortex is established. The femoral stem’s version is established by broaching parallel to the posterior cortex, which preserves the femoral stem’s native anteversion. The trial neck and ball are then fastened to the femoral stem, and LPTS is adjusted such that it is at a neutral level. The operating surgeon initiates internal rotation and applies 5–7 cm of traction to LPTS to reduce the hip. The operating surgeon directs the reduction maneuver, which is guided in with a head pusher. The traction is released, and the limb length is grossly estimated by comparing both patellae, with fluoroscopy confirming the implant positioning. The construct’s stability is evaluated by externally rotating the leg on LPS by 90° and 40° of extension. The hip joint is considered stable if the femoral head does not dislocate during this maneuver. After establishing satisfactory limb length and stability, the trial components are dislocated, and the final matching components are placed by repeating the femoral maneuvers described above. After a final fluoroscopy image (Fig. 10) has been used to validate all of the final components, closure can then be commenced. Repositioning the capsular flap is the author’s preferred method of closing it with sutures. Standard practice protocols allow the injection of local anesthetic into the joint at this stage. In the author’s experience, DAA-THA patients have good post-operative pain control [23] and are discharged within a day or two of surgery [4,24]. The fascia over the TFL is repaired, and the subcutaneous tissue is closed before the skin is closed and dressed without the use of a drain.
Broaching begins by gradually increasing the size of the broaches until an adequate fit from the medial to the lateral cortex is established. The femoral stem’s version is established by broaching parallel to the posterior cortex, which preserves the femoral stem’s native anteversion. The trial neck and ball are then fastened to the femoral stem, and LPTS is adjusted such that it is at a neutral level. The operating surgeon initiates internal rotation and applies 5–7 cm of traction to LPTS to reduce the hip. The operating surgeon directs the reduction maneuver, which is guided in with a head pusher. The traction is released, and the limb length is grossly estimated by comparing both patellae, with fluoroscopy confirming the implant positioning. The construct’s stability is evaluated by externally rotating the leg on LPS by 90° and 40° of extension. The hip joint is considered stable if the femoral head does not dislocate during this maneuver. After establishing satisfactory limb length and stability, the trial components are dislocated, and the final matching components are placed by repeating the femoral maneuvers described above. After a final fluoroscopy image (Fig. 10) has been used to validate all of the final components, closure can then be commenced. Repositioning the capsular flap is the author’s preferred method of closing it with sutures. Standard practice protocols allow the injection of local anesthetic into the joint at this stage. In the author’s experience, DAA-THA patients have good post-operative pain control [23] and are discharged within a day or two of surgery [4,24]. The fascia over the TFL is repaired, and the subcutaneous tissue is closed before the skin is closed and dressed without the use of a drain.
Learning curve
The DAA for THA is a well-known approach. However, as with any surgical technique, there is a learning curve involved. Furthermore, there are uncertainties regarding better clinical outcomes of the DAA approach than the other contemporary approaches. As a result, this approach must be scrutinized to determine whether it should be lauded as innovative or simply described as another surgical approach for THA. Multiple authors have evaluated the DAA learning curve to determine the number of cases required to become sufficient and comfortable with the technique. Nairn et al. conducted a meta-analysis of 21 studies to determine the mean operative time for surgeons using the DAA. They discovered that the average operative time by case 100 was significantly less than in case one, and the complication rate decreased significantly in later groups as the surgeon performed more cases and became familiar with the technique. Furthermore, the research found that the mean operative time began to plateau around case 100. This means that a younger or inexperienced surgeon may need to perform approximately 100 cases before demonstrating mastery of the technique [25].
DAA’s perceived benefits
With the growing popularity of DAA, it is prudent to examine its benefits compared to other acclaimed approaches. When advocating for the DAA, proponents of the approach list a slew of benefits. We will look at short- and long-term recovery, pain, in-hospital LOS, and dislocation risk to see how the DAA stacks up against other approaches.
Recovery
Proponents of the DAA argue that the DAA approach allows patients to recover faster than other approaches. Zhao et al. [26] compared the DAA to the Posterolateral (PL) approach in a randomized controlled trial of 60 patients to investigate differences in estimated blood loss, LOS, and patient reported outcomes measures ratings. Patients who received the DAA had higher Harris Hip Scores and University of California Los Angeles activity scores at 3 months. Although the long-term differences between surgical approaches are relatively minor, some surgeons use the DAA to improve short-term recovery and to focus on the patient experience during the THA procedure and episode of care.
Pain
Pain is another indicator that proponents of the DAA use as evidence and justify its usage. Pain is an ambiguous and complicated term that encompasses a wide range of conditions and classifications. Pain protocols and anesthetic use differ amongst institutions, and when comparing one method with another, the periarticular and wound anesthetic cocktails that are used may be different which may account for variances in pain experienced by patients. Although pain is a subjective measure that is difficult to quantify and standardize across populations and groups, Zhao et al. [26] compared post-operative pain in patients who underwent the DAA and PL approaches using self-reported pain scales. They found that patients who had DAA THA had less pain after 24-, 48-, and 72 h of surgery than those who had PL approach THA.
In-hospital length of stay
LOS is an important measure not only for patient safety but also for financial reasons. Reduced LOS can reduce hospital-acquired infections, lower costs, and allow for an earlier return to work or play, which can lead to improved patient satisfaction and outcomes. As a result, any procedure or advancement in treatment that reduces LOS is a highly valuable commodity. Higgins et al., reported a significant reduction in LOS when the DAA approach is used in comparison to the posterior approach in a meta-analysis of 17 studies. Despite this, they concurred that the current level of clinical evidence does not demonstrate a clear superiority of one approach over another [27].
Dislocation risk
According to some studies, the DAA may reduce the number of post-operative dislocations. However, the claims made by the DAA must be weighed against the clinical data. In a meta-analysis of 25 studies involving 7172 patients, Huerfano et al. [28] discovered no significant difference in dislocation rates between DAA and PL. On the contrary, Siljander et al. [29] discovered that the dislocation rate in DAA was lower than in the other cohorts when they examined 5341 THA procedures (3162 PL, 1846 DAA, and 333 Direct Superior). However, this finding was not statistically significant. In another study of 38,399 patients, Charney et al. found that patients treated with the DAA (n = 6428, 16.7%) had lower dislocation rates and fewer revisions for instability than those treated with the posterior approach [30].
Nerve injury
LFCN is the major nerve at risk of injury during DAA. LFCN follows a variable path around ASIS and passes through the subcutaneous tissue between the Sartorius and TFL [31]. While creating the internervous plane between the Sartorius and TFL, it is best to utilize cautious blunt dissection to prevent neuropraxia or neurolysis. Even while LFCN injuries might be frequent, they typically heal without leaving any lasting effects.
Intraoperative fracture risk
Intraoperative fractures are potentially fatal complications that can occur during primary THA. Given the risk and potentially devastating consequences of intraoperative fractures, care must be taken to evaluate the stock during broaching and implant trials. The author discovers that direct visualization of the calcar and Greater Trochanter is critical during DAA implementation to prevent varus broaching and reduce intraoperative fracture risk. Moreover, care must be given to lateralize the femur correctly to prevent varus placement and lower the chance of intraoperative fractures. DAA and its corresponding learning curve may raise the risk of intraoperative fracture in the hands of untrained practitioners. Aggarwal et al. [32] recently compared the DAA, posterior approach, a direct lateral approach, and northern approach complication rates. Among 30 intraoperative fractures identified in their dataset 10 (30%) occurred during the DAA, 14 (46%) in the posterior group, 4 (13.3%) in the northern group, and 2 (0.67%) in the direct lateral group. However, when the peri-prosthetic fracture rate in DAA was compared to the other approaches, they discovered no statistically significant difference in intraoperative fracture rate between the approaches.
Comparison of DAA THA: On table versus off table
A comparative assessment of complications associated with the DAA for THA conducted on-table versus off-table reveals intriguing insights within the surgical field. The choice between the two approaches brings forth distinct considerations that warrant examination. The on-table approach offers advantages such as improved visualization and precise anatomical alignment, potentially leading to a reduced risk of intraoperative fractures and nerve injuries. The controlled environment of the operating table supports optimal patient positioning, which may contribute to a decreased likelihood of post-surgical dislocation. Furthermore, on-table DAA allows for the strategic use of fluoroscopic guidance, potentially facilitating accurate component placement and leg length equalization [27]. The standardized setup inherent in this approach could contribute to a potentially shorter learning curve for surgical teams, possibly leading to reduced procedural errors and minimized chances of implant malposition. Conversely, off-table execution of DAA presents its own set of considerations. Challenges related to ergonomic support and consistent patient positioning may compromise visibility, potentially affecting accuracy during acetabular and femoral preparation. This could have implications for leg length discrepancies, impingement, and post-operative stability [33]. Surgeons operating off the table might face difficulties in achieving the optimal angles and depths required for component insertion, raising concerns about implant longevity and potential complications.
Drawbacks of DAA for THAx
The DAA for THA using the LPTS is not without its attendant limitations, warranting thoughtful consideration. While offering enhanced visualization and potential anatomical alignment advantages, this approach can potentially introduce challenges related to prolonged surgical time and increased intraoperative radiation exposure due to the frequent use of fluoroscopic guidance. Moreover, the requirement for precise patient positioning on the operating table may lead to increased demand for surgical staff and necessitate specialized equipment, potentially contributing to higher resource utilization. In addition, the learning curve associated with mastering the nuances of the on-table technique may impact its broader adoption, potentially posing a barrier to entry for surgeons transitioning from established methodologies.
The DAA technique, though is becoming the preferred approach by many surgeons for THA, it is associated with a significant learning curve. The author believes that using an LPTS aids in safely executing this technically demanding surgical approach in a reproducible manner, providing patients with the benefits of minimally invasive surgery, reduced pain, and hospital stay. The extension table enables controlled traction and external rotation of the lower extremity, improving the visibility of the surgical site and reducing the amount of force required during surgery. Furthermore, using an extension table allows the surgeon to perform THA in a more ergonomic position, reducing fatigue during longer procedures and can be a cutting-edge technology improving the overall experience for patients undergoing THA.
DAA has acted as a catalyst for the development and application of technical advancements that improve its efficacy and safety. Benefits come from using the LPTS rather than having an assistant manipulate the leg, assessing the component position and leg length in real time with fluoroscopy rather than mechanical guidance, and measuring leg length by feeling the patient’s ankles. The growing desire for less invasive arthroplasty with improvement in functional results makes this approach an attractive choice for surgeons in India. The LPTS may be an additional tool in reducing the learning curve associated with adopting this exciting surgical technique.
References
- 1.Wernly D, Wegrzyn J, Lallemand G, Mahlouly J, Tissot C, Antoniadis A. Total hip arthroplasty through the direct anterior approach with and without the use of a traction table: A matched-control, retrospective, single-surgeon study. J Orthop Surg Res 2021;16:45. [Google Scholar | PubMed]
- 2.Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res 2005;441:115-24. [Google Scholar | PubMed]
- 3.Berend KR, Lombardi AV Jr., Seng BE, Adams JB. Enhanced early outcomes with the anterior supine intermuscular approach in primary total hip arthroplasty. J Bone Joint Surg Am 2009;91 Suppl 6:107-20. [Google Scholar | PubMed]
- 4.Joseph NM, Roberts J, Mulligan MT. Financial impact of total hip arthroplasty: A comparison of anterior versus posterior surgical approaches. Arthroplast Today 2017;3:39-43. [Google Scholar | PubMed]
- 5.Kennon RE, Keggi JM, Wetmore RS, Zatorski LE, Huo MH, Keggi KJ. Total hip arthroplasty through a minimally invasive anterior surgical approach. J Bone Joint Surg Am 2003;85-A Suppl 4:39-48. [Google Scholar | PubMed]
- 6.Siguier T, Siguier M, Brumpt B. Mini-incision anterior approach does not increase dislocation rate: A study of 1037 total hip replacements. Clin Orthop Relat Res 2004;426:164-73. [Google Scholar | PubMed]
- 7.Woolson ST, Pouliot MA, Huddleston JI. Primary total hip arthroplasty using an anterior approach and a fracture table: Short-term results from a community hospital. J Arthroplasty 2009;24:999-1005. [Google Scholar | PubMed]
- 8.Bergin PF, Doppelt JD, Kephart CJ, Benke MT, Graeter JH, Holmes AS, et al. Comparison of minimally invasive direct anterior versus posterior total hip arthroplasty based on inflammation and muscle damage markers. J Bone Joint Surg Am 2011;93:1392-8. [Google Scholar | PubMed]
- 9.Faldini C, Perna F, Mazzotti A, Stefanini N, Panciera A, Geraci G, et al. Direct anterior approach versus posterolateral approach in total hip arthroplasty: Effects on early post-operative rehabilitation period. J Biol Regul Homeost Agents 2017;31 4 Suppl 1:75-81. [Google Scholar | PubMed]
- 10.Cohen EM, Vaughn JJ, Ritterman SA, Eisenson DL, Rubin LE. Intraoperative femur fracture risk during primary direct anterior approach cementless total hip arthroplasty with and without a fracture table. J Arthroplasty 2017;32:2847-51. [Google Scholar | PubMed]
- 11.Masonis J, Thompson C, Odum S. Safe and accurate: Learning the direct anterior total hip arthroplasty. Orthopedics 2008;31 12 Suppl 2:1417-26. [Google Scholar | PubMed]
- 12.D’Arrigo C, Speranza A, Monaco E, Carcangiu A, Ferretti A. Learning curve in tissue sparing total hip replacement: Comparison between different approaches. J Orthop Traumatol 2009;10:47-54. [Google Scholar | PubMed]
- 13.Xu Z, Zhang J, Li J, Zhang Y. Direct anterior approach in total hip arthroplasty: More indications and advantages than we found. Arthroplasty 2022;4:29. [Google Scholar | PubMed]
- 14.De Geest T, Vansintjan P, De Loore G. Direct anterior total hip arthroplasty: Complications and early outcome in a series of 300 cases. Acta Orthop Belg 2013;79:166-73. [Google Scholar | PubMed]
- 15.Jewett BA, Collis DK. High complication rate with anterior total hip arthroplasties on a fracture table. Clin Orthop Relat Res 2011;469:503-7. [Google Scholar | PubMed]
- 16.Homma Y, Baba T, Sano K, Ochi H, Matsumoto M, Kobayashi H, et al. Lateral femoral cutaneous nerve injury with the direct anterior approach for total hip arthroplasty. Int Orthop 2016;40:1587-93. [Google Scholar | PubMed]
- 17.Reichert JC, Wassilew GI, von Rottkay E, Noeth U. Compared learning curves of the direct anterior and anterolateral approach for minimally invasive hip replacement. Orthop Rev (Pavia) 2022;14:37500. [Google Scholar | PubMed]
- 18.Kennon R, Keggi J, Zatorski LE, Keggi KJ. Anterior approach for total hip arthroplasty: Beyond the minimally invasive technique. J Bone Joint Surg Am 2004;86-A Suppl 2:91-7. [Google Scholar | PubMed]
- 19.Connolly KP, Kamath AF. Direct anterior total hip arthroplasty: Literature review of variations in surgical technique. World J Orthop 2016;7:38-43. [Google Scholar | PubMed]
- 20.Ropars M, Morandi X, Huten D, Thomazeau H, Berton E, Darnault P. Anatomical study of the lateral femoral cutaneous nerve with special reference to minimally invasive anterior approach for total hip replacement. Surg Radiol Anat 2009;31:199-204. [Google Scholar | PubMed]
- 21.Barnett SL, Peters DJ, Hamilton WG, Ziran NM, Gorab RS, Matta JM. Is the anterior approach safe? Early complication rate associated with 5090 consecutive primary total hip arthroplasty procedures performed using the anterior approach. J Arthroplasty 2016;31:2291-4. [Google Scholar | PubMed]
- 22.Matsuura M, Ohashi H, Okamoto Y, Inori F, Okajima Y. Elevation of the femur in THA through a direct anterior approach: Cadaver and clinical studies. Clin Orthop Relat Res 2010;468:3201-6. [Google Scholar | PubMed]
- 23.Goebel S, Steinert AF, Schillinger J, Eulert J, Broscheit J, Rudert M, et al. Reduced postoperative pain in total hip arthroplasty after minimal-invasive anterior approach. Int Orthop 2012;36:491-8. [Google Scholar | PubMed]
- 24.Schweppe ML, Seyler TM, Plate JF, Swenson RD, Lang JE. Does surgical approach in total hip arthroplasty affect rehabilitation, discharge disposition, and readmission rate? Surg Technol Int 2013;23:219-27. [Google Scholar | PubMed]
- 25.Nairn L, Gyemi L, Gouveia K, Ekhtiari S, Khanna V. The learning curve for the direct anterior total hip arthroplasty: A systematic review. Int Orthop 2021;45:1971-82. [Google Scholar | PubMed]
- 26.Zhao HY, Kang PD, Xia YY, Shi XJ, Nie Y, Pei FX. Comparison of early functional recovery after total hip arthroplasty using a direct anterior or posterolateral approach: A randomized controlled trial. J Arthroplasty 2017;32:3421-8. [Google Scholar | PubMed]
- 27.Higgins BT, Barlow DR, Heagerty NE, Lin TJ. Anterior vs. posterior approach for total hip arthroplasty, a systematic review and meta-analysis. J Arthroplasty 2015;30:419-34. [Google Scholar | PubMed]
- 28.Huerfano E, Bautista M, Huerfano M, Nossa JM. Use of surgical approach is not associated with instability after primary total hip arthroplasty: A meta-analysis comparing direct anterior and posterolateral approaches. J Am Acad Orthop Surg 2021;29:e1126-e40. [Google Scholar | PubMed]
- 29.Siljander MP, Whaley JD, Koueiter DM, Alsaleh M, Karadsheh MS. Length of stay, discharge disposition, and 90-day complications and revisions following primary total hip arthroplasty: A comparison of the direct anterior, posterolateral, and direct superior approaches. J Arthroplasty 2020;35:1658-61. [Google Scholar | PubMed]
- 30.Charney M, Paxton EW, Stradiotto R, Lee JJ, Hinman AD, Sheth DS, et al. A comparison of risk of dislocation and cause-specific revision between direct anterior and posterior approach following elective cementless total hip arthroplasty. J Arthroplasty 2020;35:1651-7. [Google Scholar | PubMed]
- 31.Petis S, Howard JL, Lanting BL, Vasarhelyi EM. Surgical approach in primary total hip arthroplasty: Anatomy, technique and clinical outcomes. Can J Surg 2015;58:128-39. [Google Scholar | PubMed]
- 32.Aggarwal VK, Elbuluk A, Dundon J, Herrero C, Hernandez C, Vigdorchik JM, et al. Surgical approach significantly affects the complication rates associated with total hip arthroplasty. Bone Joint J 2019;101-B:646-51. [Google Scholar | PubMed]
- 33.Moslemi A, Kierszbaum E, Descamps J, Sigonney F, Biau D, Anract P, et al. Does using the direct anterior approach with a standard table for total hip arthroplasty reduce leg length discrepancies? Comparative study of traction table versus standard table. Orthop Traumatol Surg Res 2021;107:102752. [Google Scholar | PubMed]






