Distal junctional failure due to an ongoing infection is unreported. This case highlights a rare complication of a long-segment fusion surgery.
Dr. Mantu Jain, Department of Orthopedics, AIIMS, Bhubaneswar, Odisha, India. E-mail: montu_jn@yahoo.com
Introduction: Distal junctional failure (DJF) is underreported when compared to proximal junctional failure. DJF arising due to spondylodiscitis has never been reported in the literature.
Case Report: A 45-year-old lady with a body mass index of 33 presented with a long-standing inability to walk due to myelopathy secondary to continuous ossified posterior longitudinal ligament and ossified ligamentum flavum. Posterior fusion and laminectomy were done from D2 to L2. She had an initial wound breakdown with a surgical site infection, but after 6 weeks, she developed spondylodiscitis at the distal instrumented vertebra, leading to DJF. She was started on appropriate antibiotics and an extension of fusion.
Conclusion: This report demonstrates and discusses the management of a rare case of DJF arising due to spondylodiscitis of the last instrumented vertebra.
Keywords: Junctional failure, distal, spondylodiscitis, surgical site infection.
Infection after spinal instrumentation is a rare and serious complication that can occur in 2.1–8.5% of patients [1]. The challenge of managing an infection after surgical instrumentation is critical as the patient needs appropriate post-operative care and the surgeon is in a dilemma about whether to retain the implant or remove it. Long segment posterior fixation (LSPF) is required in many spinal pathologies such as tumor resection, correction of deformities, managing adult degenerative and other conditions such as ossified posterior longitudinal ligament (OPLL) and ossified ligamentum flavum (OLF) [2]. In a patient with LSPF, the ends (i.e., the proximal and distal junctions) are transitional between the instrumented and instrumented vertebrae and are sometimes prone to failure [2]. Distal junctional failure (DJF) is rare when compared to proximal junctional failure (PJF). Therefore, the cause of DFJ also remains relatively unexplored [3,4]. We present a case of DJF following spondylodiscitis in a patient who underwent LSPF for OPLL and PLF in the thoracolumbar spine.
The patient is a 45-year-old lady with a body mass index of 33 with underlying type 2 diabetes mellitus and hypertension. She was unable to walk for 2½ years and had been wheelchair bound due to lower-limb stiffness and weakness. The examination of the upper limbs was normal, but the lower limbs were hypertonic with brisk reflexes and a positive Babinski sign. Her motor could not be assessed due to her increased tone, but her sensory level was D10. The bowel and bladder were unaffected. The X-rays showed an extensive OPLL-continuous type from D2 to D12 and more pronounced in the lower thoracic area (Fig. 1a). The computed tomography scans showed OPLL that was more prominent from D8 to D12 (Fig. 1b-e). Magnetic resonance imaging (MRI) revealed multi-level compression of the spinal cord due to continuous OPLL and OLF, namely at the L1-2 level and proximally at D2-4 (Fig. 1f-i).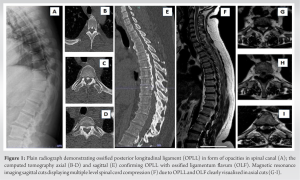 Long-instrumented decompression was done from D2 to L2 (Fig. 2).
Long-instrumented decompression was done from D2 to L2 (Fig. 2).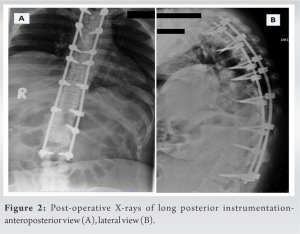 Postoperatively, her drain was removed on the 3rd day (Fig. 3a). On the 5th day postoperatively, the wound had persistent hemoserous discharge (Fig. 3b).
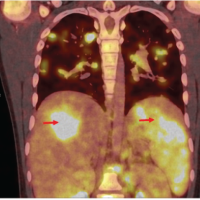
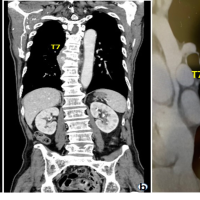
Postoperatively, her drain was removed on the 3rd day (Fig. 3a). On the 5th day postoperatively, the wound had persistent hemoserous discharge (Fig. 3b).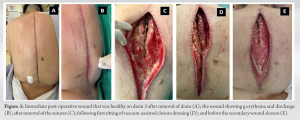 Wound debridement was done, and cultures were sent from the wound (Fig. 3c). The wound was kept open, and a negative pressure dressing in the form of vacuum-assisted closure was done. The cultures grew Klebsiella pneumonia and Morganella morganii (sensitive to cefepime, sulfamethoxazole, and trimethoprim). She was started on antibiotics, and the VAC dressing continued for another three cycles of 3–5 days each. As the wound became healthier, secondary closure was done on day 21 (Fig. 3d and e). Subsequently, she was on oral medication (sulfamethoxazole and trimethoprim) and referred to the rehabilitation team. After 3 weeks, she complained of disproportionately severe pain and deteriorating neurology. Imaging showed erosion of the L2-3 endplate, collapse of disc space, and distal functional failure (Fig. 4a and b).
Wound debridement was done, and cultures were sent from the wound (Fig. 3c). The wound was kept open, and a negative pressure dressing in the form of vacuum-assisted closure was done. The cultures grew Klebsiella pneumonia and Morganella morganii (sensitive to cefepime, sulfamethoxazole, and trimethoprim). She was started on antibiotics, and the VAC dressing continued for another three cycles of 3–5 days each. As the wound became healthier, secondary closure was done on day 21 (Fig. 3d and e). Subsequently, she was on oral medication (sulfamethoxazole and trimethoprim) and referred to the rehabilitation team. After 3 weeks, she complained of disproportionately severe pain and deteriorating neurology. Imaging showed erosion of the L2-3 endplate, collapse of disc space, and distal functional failure (Fig. 4a and b).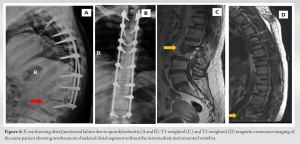 MRI confirmed spondylodiscitis at the same level without any changes in the cranial operated site (Fig. 4c and d). Revision surgery was done with debridement of disc space, and extension of fusion (after removing the L2 which had become loose) to L5 was done with a domino connector (Fig. 5).
MRI confirmed spondylodiscitis at the same level without any changes in the cranial operated site (Fig. 4c and d). Revision surgery was done with debridement of disc space, and extension of fusion (after removing the L2 which had become loose) to L5 was done with a domino connector (Fig. 5).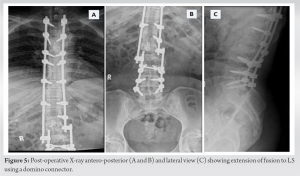 The culture was negative this time, and the previous culture was also negative for any fungal growth. Intravenous antibiotics were continued for 3 weeks until her acute phase reactants normalized and continued oral for another 6 weeks as she resumed physiotherapy. She was discharged roughly 4 months after her index surgery with some improvement in neurology. At 6-month follow-up, she had regained sensory, some power (MRC 3-4/5 in lower limbs) and her tone had reduced. Her wound was healthy though she was able to stand with support. She is on home-based physiotherapy and is on close watch for evidence of union.
The culture was negative this time, and the previous culture was also negative for any fungal growth. Intravenous antibiotics were continued for 3 weeks until her acute phase reactants normalized and continued oral for another 6 weeks as she resumed physiotherapy. She was discharged roughly 4 months after her index surgery with some improvement in neurology. At 6-month follow-up, she had regained sensory, some power (MRC 3-4/5 in lower limbs) and her tone had reduced. Her wound was healthy though she was able to stand with support. She is on home-based physiotherapy and is on close watch for evidence of union.
Junctional failure (JF) after spinal instrumentation is one of the greatest challenges for surgeons [5]. The challenge is increased manifold in the presence of infection. DFJ is underreported when compared to PFJ, and the factors contributing to DFJ have always remained elusive [3,4,6]. In the past, JF has been broadly classified either due to failure of instruments, problems at the instrument-bone interface, or accelerated adjacent disc-level degeneration. Recently, Tan et al. described another mechanism of failure of the DFJ due to a fracture of the lower instrumented vertebra [6]. The authors attributed this to increased bone fragility (osteoporosis) and stress risers (thoracolumbar or lumbosacral junction) for such failure [6-8]. A pre-existing degeneration can always facilitate JF due to altered biomechanics with such long instrumentation [9]. Spondylodiscitis leading to JF or DJF is never reported. This is a deep form of infection where our patient was at high risk (obesity, diabetes, LSPF having blood loss, immobility, and previous infection, which in our case was a superficial surgical site infection [SSI]) [1,10]. This is different from the superficial SSI, where wound drainage is the common sign that was seen in the initial post-operative period in our patient [11]. The time for diagnosing a deep SSI has been varying but longer than for a superficial SSI [11]. Unusual pain that is out of proportion should later the surgeon for any JF or infection in patients with LSPF [1,2,6]. Managing infection with implants is always a surgeon’s dilemma [1,12]. The superficial SSI can be managed with the retention of implants if there is no loosening [1]. Vacuum-assisted closure dressings can be very helpful to generate a granulating bed that can be closed secondarily as executed in our case [1]. There is a loss of correction and progressive deformities reported when instruments are removed in deep SSI [13]. Our case was unique in that only the DFJ had a deep infection and the rest of the spinal instrumented segments were not involved as seen in the radiological examination. Therefore, we needed to address the failure of DJF with extension and simultaneously get a tissue for culture and biopsy. M. morganii is a Gram-negative rod that causes various forms of infection, such as urinary tract infections, wound infections, musculoskeletal infections, and even sepsis and is reported in diabetes patients [14]. The mainstay of treatment for spondylodiscitis is always antibiotics that need to be given injectable till the acute phase reactants come to baseline and further oral antibiotics for 6–12 weeks [15].
DJF is uncommon unlike the PJF and therefore underreported. DJF arising due to spondylodiscitis was seen in our patient with multiple risk factors that required management with appropriate antibiotics and extension of fusion.
JF is a concern for spinal surgeons, especially in the setting of long posterior instrumentation. While there are some theories explaining the failure, infection is an unreported cause. The present case is an illustration of how a deep infection can lead to junctional failure.
References
- 1.Gerometta A, Olaverri JC, Bitan F. Infections in spinal instrumentation. Int Orthop 2012:457-64. [Google Scholar | PubMed]
- 2.McDonnell JM, Evans SR, Ahern DP, Cunniffe G, Kepler C, Vaccaro A, et al. Risk factors for distal junctional failure in long-construct instrumentation for adult spinal deformity. Eur Spine J 2022;31:3654-61. [Google Scholar | PubMed]
- 3.Cho KJ, Suk SI, Park SR, Kim JH, Kang SB, Kim HS, et al. Risk factors of sagittal decompensation after long posterior instrumentation and fusion for degenerative lumbar scoliosis. Spine (Phila Pa 1976) 2010;35:1595-601. [Google Scholar | PubMed]
- 4.Hosthota A, Govindasamy R, Rudrappa S. Distal junctional failure secondary to nontraumatic fracture of lower instrumented vertebra: Our experience and review of literature. Int J Spine Surg 2021;15:1031-8. [Google Scholar | PubMed]
- 5.Hostin R, McCarthy I, O’Brien M, Bess S, Line B, Boachie-Adjei O, et al. Incidence, mode, and location of acute proximal junctional failures after surgical treatment of adult spinal deformity. Spine (Phila Pa 1976) 2013;38:1008-15. [Google Scholar | PubMed]
- 6.Tan JH, Tan KA, Hey HW, Wong HK. Distal junctional failure secondary to L5 vertebral fracture-a report of two rare cases. J Spine Surg 2017;3:87-91. [Google Scholar | PubMed]
- 7.Lafage V, Ames C, Schwab F, Klineberg E, Akbarnia B, Smith J, et al. Changes in thoracic kyphosis negatively impact sagittal alignment after lumbar pedicle subtraction osteotomy: A comprehensive radiographic analysis. Spine (Phila Pa 1976) 2012;37:E180-7. [Google Scholar | PubMed]
- 8.Kwon BK, Elgafy H, Keynan O, Fisher CG, Boyd MC, Paquette SJ, et al. Progressive junctional kyphosis at the caudal end of lumbar instrumented fusion: Etiology, predictors, and treatment. Spine (Phila Pa 1976) 2006;31:1943-51. [Google Scholar | PubMed]
- 9.Arlet V, Aebi M. Junctional spinal disorders in operated adult spinal deformities: Present understanding and future perspectives. Eur Spine J 2013;22 Suppl 2:S276-95. [Google Scholar | PubMed]
- 10.Fang A, Hu SS, Endres N, Bradford DS. Risk factors for infection after spinal surgery. Spine (Phila Pa 1976) 2005;30:1460-5. [Google Scholar | PubMed]
- 11.Pull ter Gunne A, Cohen D. Incidence, prevalence, and analysis of risk factors for surgical site infection following adult spinal surgery. Spine (Phila Pa 1976) 2012;34:1422-8. [Google Scholar | PubMed]
- 12.Ho C, Skaggs DL, Weiss JM, Tolo V. Management of infection after instrumented posterior spine fusion in pediatric scoliosis. Spine (Phila Pa 1976) 32:2739-44. [Google Scholar | PubMed]
- 13.Hahn F, Zbinden R, Min K. Late implant infections caused by Propionibacterium acnes in scoliosis surgery. Eur Spine J 2005;14:783-8. [Google Scholar | PubMed]
- 14.Cetin M, Ocak S, Kuvandik G, Aslan B, Temiz M, Aslan A. Morganella morganii-associated arthritis in a diabetic patient. Adv Ther 2008;25:240-4. [Google Scholar | PubMed]
- 15.Jain M, Sahu RN, Gantaguru A, Das SS, Tripathy SK, Pattnaik A. Postoperative lumbar pyogenic spondylodiscitis: An institutional review. J Neurosci Rural Pract 2019;10:511-8. [Google Scholar | PubMed]