Primary Ewing sarcoma of the spine is a rare condition affecting the most common age group of 5–20 years, accounting for 1–3 cases/million/year. About 5 % of cases have spine involvement. Recent improvements in combination chemotherapy have improved the overall survival rates. En bloc resection and/or radiotherapy have improved local control of the disease.
Dr. Rajendra Sakhrekar, Department of Spine Surgery, The Hospital for Sick Children, Toronto, Canada. E-mail: raj.sakhrekar1@gmail.com
Introduction: Ewing sarcoma (ES) is a malignant and aggressive bony tumor affecting the most common age group of 5–20 years. It constitutes 10%–15% of all bone sarcomas and is the second most common primary malignant bone tumor after osteosarcoma. It usually presents with pain, which is typically constant and progressive in nature. The primary source of pain is due to the instability of the spine to support the weight of the body, the vertebral body’s expanding cortices due to the growing mass, compression of nerve roots due to tumor mass, pathologic fractures, spinal cord compression, and invasion of tissue by the tumor mass.
Methods: We reviewed the literature on Ewing’s Sarcoma of the spine to evaluate its etiology, clinical presentations, differential diagnosis, imaging modalities, and management with chemotherapy, radiotherapy, and surgical management. PubMed, EMBASE, Google Scholar, and Cochrane key articles were searched. Keywords such as “Ewing’s Sarcoma,” “Spine,” “etiology,” “treatment,” “surgical management,” and “en bloc resection” were used.
Discussion: The current management of Ewing’s sarcoma of the spine usually involves three primary modalities: combination chemotherapy, surgery and/or radiotherapy. Recent improvements in combination chemotherapy (vincristine, doxorubicin, cyclophosphamide±ifosfamide, and etoposide) are among the most significant factors for improving survival. Furthermore, recent advancements in radiotherapy, instrumentation, and fusion techniques in surgical management have been demonstrated to improve local disease control and overall survival.
Conclusion: Primary ES of the spine is a rare condition affecting the most common age group of 5–20 years, accounting for 1–3 cases/million/year. About 5% of cases have spine involvement. Recent improvements in combination chemotherapy have improved the overall survival rates. En bloc resection and/or radiotherapy have improved local control of the disease.
Keywords: Ewing’s sarcoma, spine, etiology, treatment, surgical management, en bloc resection.
Ewing sarcoma (ES) is a malignant and aggressive bony tumor affecting adolescents and young adults, with the most common age group being 5–20 years [1,2]. It constitutes 10%–15% of all bone sarcomas and is the second-most common primary malignant bone tumor after osteosarcoma. It may occur in extra-skeletal soft tissue in 15% of cases. It is most often diagnosed in people of Caucasian descent and occurs very rarely within the African American, Chinese, or Indian population. There is a male predominance with a male-to-female ratio of 1.5–3 to 1. It arises from unique mesenchymal progenitor cells. It is characterized by distinctive small round cell sarcoma associated with a t (11:22) translocation. The most common anatomical sites include the meta diaphysis of long bones (~50%), the pelvis (~25%), and the axial skeleton; however, it can originate in almost any bone or soft tissue [3,4,5]. ES is characterized by rapid tumor growth and extensive bone destruction.
We reviewed the literature on Ewing’s sarcoma of the spine to evaluate its etiology, clinical presentations, differential diagnosis, imaging modalities, and management with chemotherapy, radiotherapy, and surgical management. PubMed, EMBASE, Google Scholar, and Cochrane key articles were searched. Keywords such as “Ewing’s Sarcoma,” “Spine,” “etiology,” “treatment,” “surgical management,” and “en bloc resection” were used. Additional articles were identified by checking the references manually. Articles were reviewed by two independent reviewers.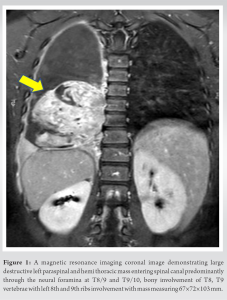
Etiology
The exact etiology of Ewing’s sarcoma is unknown; however, it is thought to be of neuroectodermal origin and no associations with environmental, genetic, familial, or radiation history have been proven. The association of t (11;22) (q24;q12) translocation is found in 85% of tumors leading to EWS-FLI-1 formation, while t (21;12) (22;12) translocations are seen in 10–15% of patients with EWS-ERG fusion formation [5].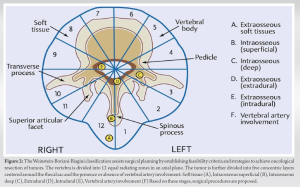
Epidemiology
Primary ES of the spine is a rare condition accounting for 1–3 cases/million/year. About 5 % involve the spine. The most common age group is 5–20 years, with approximately 30% of the cases described in children under ten and another 30% in adults over 20 [3]. There is a male predominance with a male-to-female ratio of 1.5–3 to 1. The incidence of ES in older people needs to be better described in the literature.
Clinical presentation
The presenting features are localized pain, stiffness, or swelling for a few weeks or months. Late diagnosis is expected as more than 50% of the patients present 6 months after initiation of symptoms [6]. Patients typically complain of intermittent pain that worsens at night; local erythema, mass, or swelling could also be present. Systemic symptoms, including fever and weight loss, are frequently seen and might indicate metastatic disease. The pain is usually constant and progressive in nature. The primary source of pain is due to the instability of the spine to support the weight of the body, the vertebral body’s expanding cortices due to the growing mass, compression of nerve roots due to tumor mass, pathologic fractures, spinal cord compression, and invasion of tissue by the tumor mass. Back pain, weakness in the lower extremities, sensory disturbances, and cauda equina syndrome are also seen at presentations. Kyphotic deformity with/without mechanical instability could be seen at the presentation. Anemia in the onset of ES is usually indicative of progressive disease. A complete blood count could reveal leukocytosis and elevated erythrocyte sedimentation. Constitutional symptoms, such as fever, weight loss, anorexia, and fatigue, are relatively uncommon and seen as the disease progresses. Metastatic lesions can occur in the lungs (50%), bone (25%), and bone marrow (20%) and can present with asymmetric breath sounds, pleural signs, or rales. Petechia or purpura and thrombocytopenia may be present from bone marrow metastases. A neurologic examination is of critical importance in patients with spine involvement.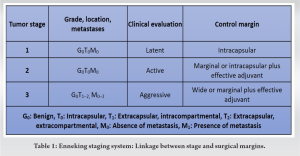
Delayed diagnosis is more common in the pelvis and axial skeleton due to the anatomic location; patients are likely to experience symptoms and notice it earlier when the tumor is even relatively small in size at the extremities and thus seek medical consultation at an earlier stage. As the pelvis and axial skeleton have large cavities, noticing the small-sized tumors is difficult. Spinal cord compression can produce neurological deficits depending on the tumor location but is often a delayed presentation. Rapidly progressing paraplegia is uncommon, and a high index of suspicion is essential for diagnosis, especially in a young patient. Although tumor size and location are debatable on prognosis, this could be one of the reasons for inferior overall survival and disease-free survival in axial and pelvic tumors, as explained in several studies.
Investigations
Primary investigations include an X-ray of the affected area demonstrating usual destructive confluent’ “moth-eaten” lesions, “Codman’s triangle” of the elevated periosteum, or multilayered “onion-skin” or “sunburst” periosteal reaction. Grossly, tumors often appear firm, gray, and friable with distinct areas of hemorrhage and necrosis. Recent guidelines from the National Comprehensive Cancer Network 2017 [7] advise imaging of primary sites should include magnetic resonance imaging (MRI) with or without computed tomography (CT), with contrast being of prime importance. Rest imaging modalities, such as CT thorax, positron emission tomography/CT, bone scan, and MRI of the spine/pelvis, to detect possible metastatic sites. MRI helps to identify soft-tissue extension, marrow involvement, and the relationship of the lesions to adjacent neurovascular structures (Fig. 1). MRI can also help to assess recurrence after tumor resection and response to neoadjuvant chemotherapy and radiation. Laboratory investigations usually demonstrate elevated erythrocyte sedimentation rate (ESR), white blood cell, and lactate dehydrogenase (LDH) with reduced hemoglobin levels. Serum LDH carries prognostic significance. A core-needle biopsy, either a fluoroscopy-guided or CT-guided or an open biopsy, is necessary to establish the diagnosis. Grossly, it may appear grayish-white with a variable amount of necrosis, hemorrhage or cyst formation, or liquid consistency mimicking pus. On histopathology, it will appear as monotonous small round blue cells with high nuclei: Cytoplasm ratio and pseudo-rosettes appearance. Immunostaining demonstrates CD99 positivity in almost 95% [7].
Classification and staging
The commonly used staging system for ES developed by Musculoskeletal Tumor Society (MSTS)/Enneking [8,9] classifies tumors by grade (low grade being stage IA-IB, high-grade stage IIA-IIB, distant metastasis stage IIIA-IIIB and subdivided by compartmental status (T1- Intra-compartmental – located in the bone cortex versus T2-extra-compartmental – extended beyond the bone cortex). Grossly, tumor size is categorized as small (≤8 cm) or large (>8 cm). Histologically, tumors are graded based on the percentage of cellular atypia – low metastatic potential tumors are classified as low-grade tumors, and low metastatic potential tumors with a higher percentage of cellular atypia are classified as high-grade tumors, for example, intramedullary osteosarcoma, Ewing’s sarcoma. The majority of Ewing’s tumors is MSTS/Enneking stage IIB or III (Table 1). The American Joint Committee on Cancer (AJCC) classification method [10] is tumor, node, metastasis by, which classifies tumors depending on tumor size, lymph node metastasis, distant metastasis, and tumor grade (cellular differentiation, mitotic rate, and extent of necrosis). The Weinstein-Boriani-Biagini classification [11] assists surgical planning for spine tumors by establishing feasibility criteria and strategies to achieve oncological resection of tumors (Fig. 2). In this system, the vertebra in the transverse plane is divided into 12 radiating zones (numbered 1 through 12 clockwise) and five circumferential layers (A through E), with A representing paravertebral and E dural involvement. This and other systems aid the surgeon in preoperative planning. Regardless of the system used, the most important criterion is the presence of a tumor-free region in the vertebra (most conveniently located in the posterior elements) whereby the spinal cord can be delivered, and the vertebra (along with the tumor and a margin of healthy tissue) can be removed. After the resection of cancer, the microscopic resection margin was defined as clear (R0) if the margin was reported as being wide or marginal and as positive (R1 or R2) if the margin was assessed as intralesional [12]. R0-Microscopic margin free of tumor cells; R1 – tumor cells microscopically present at resection margins; R2 – Tumor tissue grossly present at resection margin – seen with the naked eye.
Differential diagnosis
Differential diagnosis of ES includes other small round cell tumors such as neuroblastoma, lymphoma, neuroectodermal tumors, and synovial sarcoma. Pyogenic infection of the spine, tuberculosis, osteomyelitis, osteogenic sarcoma, and eosinophilic granuloma are the other differentials [13].
Prognostic factors
Favorable factors for survival in ES include age <10 years, tumor volume <100 mL, response to chemotherapy, and en bloc resection of the tumor. Factors indicative of more extensive disease and a worse prognosis include the presence of tumor metastasis, tumor size larger than 8 cm, pelvic location, leukocytosis, ESR, resistance to chemotherapy, elevated LDH, fever, and anemia. The prognosis of patients with disease arising in the spine is worse than those with tumors of the limbs but better as compared to patients with tumors arising in the pelvis. Histological grades have no prognostic significance.
Management
Since its first description by James Ewing in 1921 [1], management options and survival of Ewing’s sarcoma have significantly improved. The current management of Ewing’s sarcoma of the spine usually involves three primary modalities: combination chemotherapy, surgery and/or radiotherapy [13]. A multidisciplinary and interprofessional team approach is essential to achieve the best results in young patients of Ewing’s sarcoma. The skilled team should include pediatric oncologists, radiologists, orthopedic surgeons, radiation oncologists, pathologists, and pharmacists for the best outcomes. Recent improvements in combination chemotherapy (vincristine, doxorubicin, cyclophosphamide ± Ifosfamide, and etoposide) are among the most significant factors for improving survival [14-17]. Furthermore, recent advancements in radiotherapy, instrumentation, and fusion techniques in surgical management have been demonstrated to improve local disease control and overall survival. Radiotherapy is helpful as a mode of local therapy. However, in spine tumors, consider its proximity to the spinal cord. In the lumbar region, the renal structures, its use is restricted in dose [≥ 50.4 Gy] and extent to reduce radiation-induced complications [15]. Recent research studies by the AO spine tumor oncology group suggested that en bloc resection may provide improved local control for Ewing’s sarcoma of the spine but not improved overall survival. They also recommended that RT be used for local control alone or to supplement incomplete resection [4]. Regarding resection margins, an en bloc resection is defined as a surgical resection aiming to excise a tumor, fully covered by a continuous shell of healthy tissue called the “margin.”
A few terms regarding surgical resection need to be mentioned here:
- Intralesional excision: The resection will be called “intralesional” when the surgeon incidentally or intentionally violates the tumor. Intentional intralesional resection comes in scenarios where a surgical margin requires resection of functional tissues such as a nerve, nerve sheath, dura, thoracic duct, or a major vessel as it lies close to the tumor or is infiltrated by the tumor or the patient presents with acute neurological deficit secondary to epidural compression [10, 11, 12, 18-20].
- Marginal resection refers to the gross total removal of the tumor with the capsule intact, but no effort was made to take a rim of surrounding normal tissue.
- Wide resection refers to the gross total removal of the tumor and a cuff of normal surrounding tissue circumferentially.
- Radical resection refers to en bloc removal of a tumor along with the entire compartment of origin. This is rarely achieved in the spine without an unacceptable level of morbidity (e.g., hemicorporectomy for a distal sacral chordoma).
- En bloc resection with negative tumor margins should be the surgical goal for ES. Subtotal or partial resection should be avoided. The decision to sacrifice the structure depends on the risk of local recurrence and the impact on the outcome versus the functional impairment. From an oncologic point of view, if wide-margin resection is essential to accomplish regional and systemic cure of the disease, then the surgeon should carefully assess and choose to disregard the functional role of tissue. The more aggressive the tumor, the more critical it becomes to obtain a sufficient margin. For more aggressive tumors, it is essential to assess the tumor control and long-term survival option over sacrificing critical anatomical structures, and even paraplegia can be acceptable to achieve an oncologically appropriate resection. Furthemore, it is to be noted by the principle that while dealing with a benign tumor or metastasis, it is not advisable to sacrifice functionally important structures like nerve roots as the management aims to improve or preserve function without unnecessary morbidity [18].
The decision-making process and understanding of the patient and guardians for the surgical procedure are vital when functional loss is expected. Their experience, acceptance, and consent of the functional loss are necessary to execute the plan for disease control and long-term survival. The full extent of the surgical management of each vertebral segment is beyond the scope of this review article, although we tried to review it here briefly [21,22].
- The cervical Ewing’s Sarcoma: The cervical spine has peculiar anatomy, with special mention of the presence of the vertebral artery (typically between C6-atlas). The resection of the tumor needs to be carefully planned. The surgeon needs to look for dominant versus non-dominant vertebral arteries. Tumor resection must be careful if it requires the sacrifice of the vertebral artery or precautionary measures should be taken if it gets damaged. The involvement of a neurovascular surgeon, vascular surgeon, or interventional radiologist during surgical planning could prevent damage to this structure, can avoid catastrophic bleeding, and can also prevent devastating strokes in the brainstem.
Regarding resection of cervical nerve structures, the C2 and C3 nerve roots can typically be sacrificed without much morbidity; some patients might complain of occipital numbness, but overall, it is well tolerated. C3, 4, and 5 nerve root damage or sacrifice leads to ipsilateral diaphragmatic paralysis. C5 and C6 nerve root damage or sacrifice affect the deltoid and biceps strength, respectively, and weakness in these muscles is poorly tolerated. C7 nerve root damage or sacrifice affects the triceps strength, and its sacrifice tolerance is somewhat better than C5 and C6 nerve root sacrifice. C8 and T1 root damage or sacrifice affect intrinsic hand muscles and significantly impair fine hand motor function. As discussed above, in the cervical spine, in the presence of an anterior vertebral tumor, sacrifice of the nerve roots is usually associated with significant morbidity, and careful dissection of the tumor with an anterior approach can be obtained with the preservation of the nerve roots and spinal cord. Reconstruction can be done of the anterior load-sharing column with either anterior structural bone grafts, mesh cages, or expandable cages with the restoration of the posterior tension band, with multi-level instrumentation via a posterior approach.
- Thoracic Ewing’s Sarcoma: The thoracic spine tumor can be comprehensively approached posteriorly or laterally. The ribs and nerve roots can be sacrificed without much debilitation. The T2-12 nerve root sacrifice can cause some chest wall numbness, which typically resolves. Furthermore, the thoracic rib cage itself provides additional stability to this segment. Once the ribs are resected laterally, the surgical corridor from a posterior approach becomes quite comprehensive, and most operations can be done in a single stage in the prone position. In the case of two-stage surgery, in the first stage, the neural elements to be protected are dissected free from the surrounding tissues, and a window is created where the spinal cord and/or spinal nerves can be delivered. In the second stage, the tumor and accessible margin of healthy tissue are dissected circumferentially, and the specimen is removed. The appropriate implants are inserted, and reconstruction is achieved accordingly.
- Lumbar Ewing’s sarcoma: The principles of resection and reconstruction remain constant for the lumbar cervical and thoracic spines. The sacrifice of the lumbar nerve roots (including S1) results in significant lower extremity weakness and typically should avoided. In cases where sacrifice is necessary, plastic surgeons’ reconstruction of the lumbar nerve roots with sural nerves should be considered and planned during the same stage of the surgery.
- Sacral Ewing’s sarcoma: En bloc resection of sacral tumors is technically challenging, needing pelvic fixation and reconstruction. Bacci et al. demonstrated that gross total resection with negative margins can obtain local control and more prolonged survival. They also suggested that intralesional excision combined with radiation and chemotherapy was less effective than radiation and chemotherapy.
A recent study by Harrop et al. [21] in aggressive osteoblastomas and giant cell tumors of the thoracic and lumbar spine demonstrated that en bloc resection is strongly recommended to minimize the risk of local recurrence when anatomically achievable. In 1992, Sharafuddin et al. [22] demonstrated Ewing’s sarcoma case series of 7 patients and described six patients treated surgically, of which 4 had laminectomies with tumor excision, 1 had anterior decompression with tumor excision, and 1 had en bloc tumor excision. Furthermore, all seven patients were treated with systemic chemotherapy (VAC-A); except for one patient, six received radiotherapy too. Local recurrence was noted in a patient who had undergone laminectomy and adjuvant radiotherapy. Three patients died of disease at 6 and 10 months, and a 3rd patient died of urosepsis. The disease-free survival in the patient who underwent en bloc resection was 7 months. In 2002, Talac et al. [23] analyzed 30 primary spine sarcomas, with 7 cases of Ewing’s sarcoma. Of 7 patients, five underwent piecemeal excision, and 1 5 had developed local recurrence (20%). Rest 2 patients had en bloc resections and did not demonstrate local recurrence. The disease-free survival for the Ewing’s sarcoma subgroup was unreported. In 2011, Boriani et al. [11,24] studied 27 patients treated with systemic chemotherapy combined with radiotherapy and/or surgery in three different periods: 1979–1982, 1983–1990 and 1991–2008. The study suggested tumor-free margin en bloc resection provided better local control and more prolonged survival, whereas the results of intralesional resection were worse than chemotherapy and radiotherapy alone. Hesla et al. [23] in 2018, retrospectively reviewed 24 patients diagnosed with Ewing’s sarcoma between 1986 and 2012 through the Scandinavian sarcoma group registry. 19/23 patients had neurologic changes at the time of presentation. Regardless of whether emergency decompression surgery was performed, 13/19 patients recovered completely from their neurological condition. Definitive radiotherapy was the primary mode of care in 18/24 of the patients. 13/24 patients underwent spinal decompressive surgery due to spinal cord compression. The study concluded that urgent decompressive surgery without establishing the histological diagnosis might increase the risk for local recurrence and also suggested that urgent decompressive surgery does not have any clear advantage over non-surgical treatment in terms of neurologic recovery. Several studies suggested decompression surgery of symptomatic compression of the spinal cord for the prevention of major neurological deficits [22,25]. However, more evidence still needs to be provided to guide the optimal therapeutic approach in primary spinal ES. The highly chemosensitive nature of Ewing’s sarcoma, responding to chemotherapy with a reduction of the disease burden and consequently decreasing spinal cord compression, suggests avoiding primary surgery as immediate primary surgical decompression may have a negative effect on the future perspective of a chemosensitive ES. It is also to be emphasized that the upfront surgery may be considered a definitive way to decompress the spine in the presence of neurological symptoms, given that the same result is difficult achievable with chemotherapy. Either chemotherapy first followed by surgery or immediate primary surgery followed by chemotherapy. Both approaches aim for the local control of the ES and achieve the primary goal of the treatment; the literature evidence is still lacking and needs to be addressed in future [21,22,25,26].
Primary ES of the spine is a rare condition affecting the most common age group of 5–20 years, accounting for 1–3 cases/million/year. About 5% of cases have spine involvement. Recent improvements in combination chemotherapy have improved the overall survival rates. En bloc resection and/or radiotherapy have improved local disease control.
Primary ES of the spine is an uncommon illness that affects 1-3 instances per million people annually and is most common in the age bracket of 5-20 years. Roughly 5 percent of cases affect the spine. Recently, combined chemotherapy has shown advancements that have raised overall survival rates. Local disease control has been enhanced by radiation and/or en bloc resection.
References
- 1.Ewing J. The classic: Diffuse endothelioma of bone. Proceedings of the New York pathological society. 1921;12:17. Clin Orthop Relat Res 2006;450:25-7. [Google Scholar | PubMed]
- 2.Ludwig JA. Ewing sarcoma: Historical perspectives, current state-of-the-art, and opportunities for targeted therapy in the future. Curr Opin Oncol 2008;20:412-18. [Google Scholar | PubMed]
- 3.Esiashvili N, Goodman M, Marcus RB Jr. Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: Surveillance epidemiology and end results data. J Pediatr Hematol Oncol 2008;30:425-30. [Google Scholar | PubMed]
- 4.Sciubba DM, Okuno SH, Dekutoski MB, Gokaslan ZL. Ewing and osteogenic sarcoma: Evidence for multidisciplinary management. Spine (Phila Pa 1976) 2009;34:S58-68. [Google Scholar | PubMed]
- 5.Lessnick SL, Ladanyi M. Molecular pathogenesis of Ewing sarcoma: New therapeutic and transcriptional targets. Annu Rev Pathol 2012;7:145-59. [Google Scholar | PubMed]
- 6.Widhe B, Widhe T. Initial symptoms and clinical features in osteosarcoma and Ewing sarcoma. J Bone Joint Surg Am 2000;82:667-74. [Google Scholar | PubMed]
- 7.Martínez MA, Garcí RN, Galván JJ, Marrero OB, Castro IG. Ewing’s sarcoma: Histopathological and immunohistochemical study. Orthopedics 2003;26:723-5. [Google Scholar | PubMed]
- 8.Nogueira Drumond JM. Efficacy of the enneking staging system in relation to treating benign bone tumors and tumor-like bone lesions. Rev Bras Ortop 2010;45:46-52. [Google Scholar | PubMed]
- 9.Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res1986;204:9-24. [Google Scholar | PubMed]
- 10.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 2017;67:93-9. [Google Scholar | PubMed]
- 11.Chan P, Boriani S, Fourney DR, Biagini R, Dekutoski MB, Fehlings MG, et al. An assessment of the reliability of the enneking and weinstein-boriani-biagini classifications for staging of primary spinal tumors by the spine oncology study group. Spine (Phila Pa 1976) 2009;34:384-91. [Google Scholar | PubMed]
- 12.Kandel R, Coakley N, Werier J, Engel J, Ghert M, Verma S. Surgical margins and handling of soft-tissue sarcoma in extremities: A clinical practice guideline. Curr Oncol 2013;20:e247-54. [Google Scholar | PubMed]
- 13.Durer S, Gasalberti DP, Shaikh H. Ewing Sarcoma. Treasure Island, FL: StatPearls Publishing; 2023. [Google Scholar | PubMed]
- 14.Paulussen M, Craft AW, Lewis I, Hackshaw A, Douglas C, Dunst J, et al. Results of the EICESS-92 study: Two randomized trials of Ewing’s sarcoma treatment--cyclophosphamide compared with ifosfamide in standard-risk patients and assessment of benefit of etoposide added to standard treatment in high-risk patients. J Clin Oncol 2008;26:4385-93. [Google Scholar | PubMed]
- 15.Schuck A, Ahrens S, Paulussen M, Kuhlen M, Könemann S, Rübe C, et al. Local therapy in localized Ewing tumors: Results of 1058 patients treated in the CESS 81, CESS 86, and EICESS 92 trials. Int J Radiat Oncol Biol Phys 2003;55:168-77. [Google Scholar | PubMed]
- 16.Bacci G, Longhi A, Briccoli A, Bertoni F, Versari M, Picci P. The role of surgical margins in treatment of Ewing’s sarcoma family tumors: Experience of a single institution with 512 patients treated with adjuvant and neoadjuvant chemotherapy. Int J Radiat Oncol Biol Phys 2006;65:766-72. [Google Scholar | PubMed]
- 17.Womer RB, West DC, Krailo MD, Dickman PS, Pawel BR, Grier HE, et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: A report from the children’s oncology group. J Clin Oncol 2012;30:4148-54. [Google Scholar | PubMed]
- 18.Nogueras JJ, Jagelman DG. Principles of surgical resection. Influence of surgical technique on treatment outcome. Surg Clin North Am 1993;73:103-16. [Google Scholar | PubMed]
- 19.Lu M, Zhou Z, Chen W, Lei Z, Dai S, Hou C, et al. En bloc resection of huge primary tumors with epidural involvement in the mobile spine using the “rotation-reversion” technique: Feasibility, safety, and clinical outcome of 11 cases. Front Oncol 2022;12:1031708. [Google Scholar | PubMed]
- 20.Tomita K, Kawahara N, Murakami H, Demura S. Total en bloc spondylectomy for spinal tumors: Improvement of the technique and its associated basic background. J Orthop Sci 2006;11:3-12. [Google Scholar | PubMed]
- 21.Ozturk AK, Gokaslan ZL, Wolinsky JP. Surgical treatment of sarcomas of the spine. Curr Treat Options Oncol 2014;15:482-92. [Google Scholar | PubMed]
- 22.Boussios S, Hayward C, Cooke D, Zakynthinakis-Kyriakou N, Tsiouris AK, Chatziantoniou AA, et al. Spinal Ewing sarcoma debuting with cord compression: Have we discovered the thread of ariadne? Anticancer Res 2018;38:5589-97. [Google Scholar | PubMed]
- 23.Talac R, Yaszemski MJ, Currier BL, Fuchs B, Dekutoski MB, Kim CW, et al. Relationship between surgical margins and local recurrence in sarcomas of the spine. Clin Orthop Relat Res 2002;397:127-32. [Google Scholar | PubMed]
- 24.Boriani S, Amendola L, Corghi A, Cappuccio M, Bandiera S, Ferrari S, et al. Ewing’s sarcoma of the mobile spine. Eur Rev Med Pharmacol Sci 2011;15:831-9. [Google Scholar | PubMed]
- 25.Gopalakrishnan CV, Shrivastava A, Easwer HV, Nair S. Primary Ewing’s sarcoma of the spine presenting as acute paraplegia. J Pediatr Neurosci 2012;7:64. [Google Scholar | PubMed]
- 26.Vogin G, Helfre S, Glorion C, Mosseri V, Mascard E, Oberlin O, et al. Local control and sequelae in localised Ewing tumours of the spine: A French retrospective study. Eur J Cancer 2013;49:1314-23. [Google Scholar | PubMed]











