1. Charcot arthropathy is a slowly progressive and chronic joint disease seen in patients with neurosensory deficit. 2. Diabetes mellitus (DM) is the most common cause of neuropathy in Charcot arthropathy. 3. Foot ulcer is one of the many complications that can arise from nerve damage caused by diabetes. 4. Major lower extremity amputation is often the end-line treatment.
Dr. Angelo V Vasiliadis, 2nd Orthopaedic Department, General Hospital of Thessaloniki "Papageorgiou", 56429 Thessaloniki, Greece /Department of Orthopaedic Surgery and Sports Medicine, Croix-Rousse Hospital, 69002 Lyon, France. E-mail: vasiliadis.av@gmail.com
Introduction: Charcot arthropathy consists of a rapid and destructive complication of the joints following the loss of innervation caused by many complicated etiologies. Diabetic neuropathy has become the most common etiological factor.
Case Report: We present a case of a 64-year-old female patient with a history of chronic renal failure on hemodialysis, hypertension, hypothyroidism, and Type 2 diabetes, complicated with neuropathy and Charcot disease, who referred to our department. Initially, the patient was managed with a restraint orthotic device due to a bimalleolar ankle fracture. An unsuccessful treatment and the presence of a pressure ulcer with pus-like drainage on the lateral malleolus 2 months later led to the decision for a below-knee amputation.
Conclusion: High clinical suspicion by the attending physician may reduce the risk of complications and lead to proper treatment with better outcomes.
Keywords: Charcot arthropathy, bimallelolar fracture, diagnosis, treatment, amputation.
It is well known that Charcot arthropathy represents a potentially devastating complication and is most frequently encountered in the diabetic population [1]. This progressive and inflammatory condition is triggered by a combination of mechanical, vascular, and biological factors, which can lead to the destruction of joints and surrounding bony structures [1,2]. Patients with severe or unstable deformities, late diagnosis, and incorrect treatment can lead to major amputations, resulting in changes in the patient’s lifestyle and quality of life [2]. Thus, the purpose of the present study is to describe a complicated case of Charcot arthropathy after an unstable ankle fracture and to propose a new treatment algorithm based upon the clinical and radiological findings.
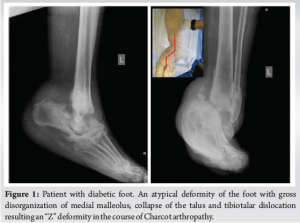
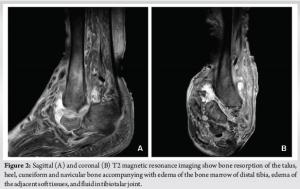
A 64-year-old obese woman (body mass index of 43.6 kg/m2) with a medical history of chronic renal failure on hemodialysis, hypertension, hypothyroidism, and uncontrolled type 2 diabetes was referred to our department with complaints of ankle pain and an inability to bear weight on the left limb for the past 2 months. Physical examination revealed an atypical deformity of the foot (Fig. 1), ligament deficiency, bony prominence, and a pressure ulcer (1.7 cm × 2.5 cm in diameter) over the lateral malleolus, as seen in Charcot arthropathy. Plain radiographs showed an equinovarus ankle deformity with supinated feet (Fig. 1). Magnetic resonance imaging revealed bone resorption of the talus, calcaneus, cuneiform, and navicular bones, accompanied with damaged soft tissues and fluids in the tibiotalar joint (Fig. 2). In addition to the above, the imaging showed non-union on the grounds of a preceding bimalleolar fracture that was addressed conservatively. Operative treatment was proposed 6 months ago due to a low-energy ankle fracture, but the patient denied surgery and was treated conservatively with a restraint orthotic walker.
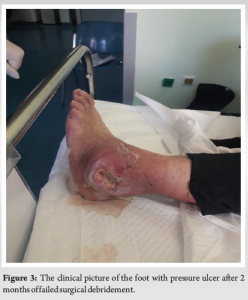
In close cooperation with the attending nephrologist and the diabetologist at our center, further investigations were ordered. Laboratory reports were a leucocyte count of 18,000/μL, an erythrocyte sedimentation rate of 84 mm/h, a C-reactive protein of 98 mg/L, and a hemoglobin A1c (HbA1c) of 10.5, indicating poor control of her glucose levels. The pressure ulcer underwent an initial debridement, in which all non-viable and infected tissues were resected and washed repeatedly with large quantities of saline solution. Deep cultures were taken and sent for culture and sensitivity tests. After the initial debridement, broad-spectrum antibiotics were initiated and then adjusted for more specific coverage as culture samples became available, indicating a Staphylococcus aureus infection. Unfortunately, after 2 months, it was evident that conservative treatment had failed to alleviate the clinical symptoms. Over the next few days, she developed increasing purulent drainage from a remarkably big ulcer measuring 2.5 cm × 4.8 cm, as well as erythema, swelling, and other signs of infection, which were tracking up the malleolus (Fig. 3). Following thorough discussions on treatment modalities with the patient, it was decided on surgical intervention. An amputation was originally recommended, and the patient underwent a below-knee amputation.
After the procedure, the patient came weekly for a routine follow-up along the 1st month post-operatively. Every follow-up appointment included thorough wound care and a clinical and radiographical evaluation. Six-week post-operatively, a prosthetic limb device was placed. Three months postoperatively, the patient achieved functionality, autonomy, and social interaction for her age.
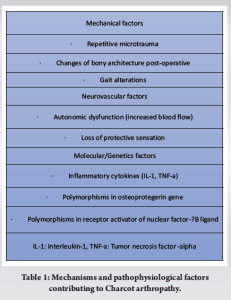
Charcot arthropathy is a chronic, progressive disease affecting the bones, joints, and soft tissues, most commonly occurring in the foot and ankle as a result of diabetic peripheral neuropathy [1]. Furthermore, trauma is shown to play a major role in the pathway of Charcot deformity in diabetic patients [3]. The actual prevalence of Charcot arthropathy is unknown due to the fact that the true cause is not discovered until later [4]. However, several population-based studies have reported an estimated prevalence, which varies between 0.3 and 13% in patients with DM [4-6]. This frequency seems to increase when diabetes is complicated by peripheral neuropathy. O’Loughlin et al. reported a prevalence of 0.3% in an observational retrospective study, while the majority of patients were male (68%) and had Type II DM (73%) [6]. A retrospective study in the endocrinology department of a South Indian tertiary hospital analyzed the clinical and radiological data of 1475 patients with Type 2 DM and showed that 144 (9.8%) patients had Charcot arthropathy [7]. Similarly, Fauzi et al. reported an increased incidence of Charcot arthropathy in patients who have a history of foot problems, with the most common anatomical site of deformity in the midfoot (45.8%), followed by ankle joints (22.9%) and multiple sites (16.7%) [8]. Charcot arthropathy is a main complication of DM, and despite the considerable progress in diabetes treatment, the long-term consequences of this disease still increase mortality, morbidity, and lower quality of life [2,8]. The theories regarding the pathophysiology state that gross bony disorganization is based on the loss of pain sensation combined with repetitive microtrauma (neurotraumatic theory), while joint destruction is secondary to an autonomic dysfunction with arteriovenous shunting and increased arterial perfusion, leading to bone resorption and weakening (neurovascular theory) [9]. Furthermore, some other factors have been correlated with the pathogenesis of Charcot arthropathy. Studies have suggested that polymorphisms in the osteoprotegerin gene (OPG) and in the receptor activator of nuclear factor-κB ligand (RANKL) are also predisposing factors for Charcot arthropathy by an increasing ratio of RANKL/OPG in the blood serum, resulting in increased bone resorption due to RANKL-induced osteoclastogenesis [10,11]. Furthermore, Charcot arthropathy is characterized by an excessive local inflammatory response linked to a possible neuropathic dysregulation of the inflammatory reflex and a continual production of pro-inflammatory cytokines such as interleukin-1 and tumor necrosis factor alpha, which can further promote the expression of RANKL [11,12]. This activity can affect the balanced process of bone remodeling and lead to osteolysis and bone breakdown (Table 1) [12].
In clinical practice, Charcot arthropathy can be classified into the acute and chronic phases [4]. In the acute active phase, patients generally present with a painless swelling of the foot and ankle, usually warm, which is warmer than the contralateral side, and with erythema, which is sometimes confused with an active infection. In the chronic inactive phase, signs of inflammation gradually recede and disappear, while deformities may develop, such as collapse of the longitudinal arch with rocker bottom deformity and a prominent medial aspect of the midfoot [2,4].
Early recognition and diagnosis, which are essential for an early off-loading treatment, are fundamental prerequisites to achieve the best outcomes and reduce the possibility of recurrence of the disease [6,13]. The effectiveness of the treatment is maximized when it begins as early as possible [13]. A total contact cast continues to be the first-line treatment for Charcot without fracture to avoid the formation of pressure ulcers and changes in bone [14]. As bone marrow edema is self-limiting, off-loading of the affected region can be continued until a significant decrease in bone marrow edema is observed in magnetic resonance imaging (MRI); then the cast can be discontinued and an orthopedic shoe can be adapted [13].
Nevertheless, the management of an ankle fracture in a patient with complicated DM can be more complex. As stated before, trauma is shown to be a valid predictor of Charcot onset when DM is pre-existing. Thus, the high suspicion index is the first-line tool for the clinician when evaluating a diabetic patient with an acute ankle injury. It is shown in recent studies [3,15,16] that very rigid fixation of an ankle fracture or even primary arthrodesis is linked to better outcomes regarding Charcot onset, union rates, infection risk, earlier mobilization, and prevention of amputation. On the contrary, conservative treatment of foot and ankle fractures is associated with complication rates that vary from 75% to 100% in the literature [15,16], with malunion or non-union being the most likely. In addition, amputation rates specifically can be considerably higher in conservatively treated patients [15].
Casting therapy and off-loading of the affected limp cannot address the subsequent bone destruction due to osteolysis [17]. Additional to these, the medical management of Charcot arthropathy includes treatment with bisphosphonates (BPs), calcitonin, and Vitamin D supplementation [18,19]. Studies have shown increased levels of bone resorptive markers in patients with acute Charcot arthropathy. These findings guided clinicians to use BPs and calcitonin along with off-loading and immobilization in the acute phase [6,17]. Nowadays, anti-resorptive agents like BPs have been used in different studies and have been shown to be effective by reducing bone turnover markers and skin temperature [20,21]. However, they have not been shown to shorten the immobilization time [22] or reduce the deformity and ulceration [6]. For patients with Charcot arthropathy and DM, gastroparesis and vascular complications, including hypertension and coronary artery disease, are possible to be expected within years of the disease. The adverse effects and contra-indications of BPs should be kept in mind when treating such patients in the acute phase of the disease [17]. Similarly, the use of calcitonin and Vitamin D has been reported as adjunct treatments to conventional therapy [18,19]. Calcitonin inhibits bone resorption by its direct action on the osteoclast receptors and seems to prevent the progression of acute Charcot arthropathy [17]. Furthermore, a positive analgesic effect has been reported through central and peripheral mechanisms [23].
Despite these non-operative first-line treatment modalities, deformities, ulceration, infection, and amputation are possible, and sometimes they cannot be avoided for chronic cases [22,24]. The ulcers may develop at sites exposed to repetitive high pressures and in anatomical regions of residual deformities [24]. First-line treatment with off-loading and cast immobilization can prevent the formation or allow the healing of ulcers and changes in bones [14]. However, surgical treatment with simply removal of the bony prominence may be required when conservative treatment fails [19]. It is obvious that the prevention of ulceration is crucial to reduce the risk of subsequent amputation [6] and achieve the preservation of quality of life [14].
Since the early stages of Charcot may be indistinguishable from other pathologies characterized by erythema, swelling, and elevated skin temperature (more than 2°C compared to the non-affected limb), similar to the clinical presentation of cellulitis, deep venous thrombosis, and osteomyelitis, a weekly assessment of the limb is required [15,25]. Regular appreciation of the soft-tissue condition, vascular capacity, and radiological assessment of the bony structures and joints are the keystones of the follow-up. Clinically, it is also important to diagnose or exclude infection [24]. Conventionally, the diagnosis of infectious diseases relies on serologic assays and cultures [26], and it is usually easy to rule out when there is no active or history of foot ulceration [24]. According to previous studies, risk factors for infection and osteomyelitis include ulceration, peripheral neuropathy, vascular disease, foot deformity, a history of previous amputations or foot surgical procedures, and immunosuppression [8, 27-29]. Infection is a serious complication of Charcot arthropathy and poses great and significant difficulties in the patient’s management [29]. In those cases, irrigation, debridement, and antibiotics are still the standard of care and are usually recommended as first-line treatments in the acute phase [29-31]. In addition to these, adequate control of blood sugar (HbA1c <7%) and management of any malnutrition issues, such as low albumin and Vitamin D levels, are also of great importance, especially when operative treatment is demanded. In this phase, immobilization can be continued until the resolution of symptoms. In a web-based observational study, Game et al. reported a median duration of immobilization of 9 months until complete resolution of symptoms, depending on the use of the off-loading device [32].
However, in some cases, the infection is extensive and cannot be limited, leading to osteomyelitis, which can be difficult to manage [32]. Bone can be infected through the hematogenous route, contiguous spread from nearby tissue, or due to direct inoculation of bone from trauma [13,33]. The prevalence of osteomyelitis in the diabetic population is between 10% and 20%, and this prevalence may be as high as 66% when clinically severely infected foot ulcers are present [34]. Radiographic findings of osteomyelitis range from osteopenia and lytic lesions to cortical destruction and periosteal reaction at the onset of the disease, while inhomogeneous osteosclerosis and/or sequestrum formation are characteristic of chronic conditions [2]. If there is no radiographic evidence of osteomyelitis, diagnosis can be confirmed by MRI due to its diagnostic accuracy, especially in the early stages of the disease [24].
The treatment of osteomyelitis in Charcot arthropathy is complex and demanding from both sides, patients and physicians [28]. Treatment strategies vary greatly depending on the stage of the disease and the presence of any comorbidity [33]. People with diabetes are more prone to develop osteomyelitis as a result of foot ulceration, which can negatively affect the antibiotic delivery and finally affect prognosis of the disease [28,33]. Impaired healing in diabetes and immunocompromised populations is the result of a complex pathophysiological mechanism involving peripheral vascular disease, which contributes to a poor and slow healing rate and also affects the clinical course and outcomes of osteomyelitis [33]. The risk of complications and treatment failure seems to be high if diagnosis is initially missed or if the conservative treatment is delayed. In these cases, the overall strategy for surgical management may be more aggressive and extensive to achieve better functional outcomes and limit the possibility of amputation [33,35]. However, lower extremity amputation seems to be a more reliable solution in 20% of diabetic patients with osteomyelitis [33].
All of the above lead to the need for a multidisciplinary team approach effectively to address all aspects of those challenging cases of diabetic patients with Charcot deformities either found solely or in combination with an ankle fracture. A close cooperation of the diabetologist, radiologist, and nutritionist with the attending orthopedic surgeon and, depending on other relevant comorbidities, with the vascular specialist and nephrologist is necessary to make patient-specific decisions toward the optimal outcome and prevention of devastating interventions such as an amputation.
Accordingly, to improve functional outcomes, physicians must spend more time with patients. Patient education is an important component of Charcot arthropathy management and aims to provide adequate and relevant information with the goal of increasing understanding of the disease [29]. It is crucial for the patient to understand the nature of this limb-threatening condition, the proposed treatment for it, and the disastrous consequences, including amputation, if Charcot arthropathy remains unrecognized or improperly managed [29,36]. Compliance with strict immobilization in the first stage of the disease and regular follow-up by each attending physician may improve the prognosis and outcomes of Charcot arthropathy [28,29].
The treatment of Charcot arthropathy is mostly conservative, with immobilization and off-loading in the active phase [14]. It is important to note that surgical treatment is considered only if conservative treatment has not been successful [22]. Surgery has generally been advised for resecting infected bone (osteomyelitis), removing bony prominences, or correcting deformities that could not be successfully accommodated with therapeutic footwear. In the case of unrecognized or improperly managed Charcot arthropathy, can have severe consequences, including amputation [29,36]. Developing ulcers in Charcot arthropathy increases the risk of major amputation [6], and patients with concomitant neuropathy, diabetes, and severe foot deformities and associated with peripheral arterial disease also have a major risk of ulceration and infection [8,29]. The risk of amputation also increases in patients previously treated for foot ulceration [28]. As a result, clinicians have a pivotal role in early recognition and prompt treatment to avoid these consequences. In addition, a lifelong program of patient education and regular foot care are required as integral aspects [29]. Fig. 4 shows the authors’ recommended treatment algorithm for the management of Charcot arthropathy.
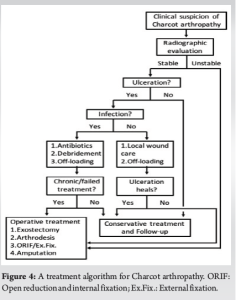
This case highlights the importance of a high index of clinical suspicion for Charcot arthropathy and the need for early recognition and intervention to avoid deformity, decreased function of the lower extremity, and the devastating consequences of amputation. In addition, the proposed treatment algorithm provides a simple and well-organized protocol that can effectively guide physicians and also positively affect the prognosis of the treatment.
The diagnosis and appropriate treatment of Charcot arthropathy are primarily dependent on the initial clinical presentation and therefore require high clinical suspicion by the attending physician for all patients with diabetes and neuropathy who present with clinical signs and symptoms.
References
- 1.Rogers LC, Frykberg RG, Armstrong DG, Boulton AJ, Edmonds M, Van GH, et al. The Charcot foot in diabetes. Diabetes Care 2011;34:2123-9. [Google Scholar | PubMed]
- 2.Konarzewska A, Korzon-Burakowska A, Rzepecka-Wejs L, Rzepecka-Wejs L, Sudoł-Szopińska I, Szurowska E, et al. Diabetic foot syndrome: Charcot arthropathy or osteomyelitis? Part I: Clinical picture and radiography. J Ultrason 2018;18:42-9. [Google Scholar | PubMed]
- 3.Gandhi A, Liporace F, Azad V, Mattie J, Lin SS. Diabetic fracture healing. Foot Ankle Clin 2006;11:805-24. [Google Scholar | PubMed]
- 4.Frykberg RG, Belczyk R. Epidemiology of the Charcot foot. Clin Podiatr Med Surg 2008;25:17-28, V. [Google Scholar | PubMed]
- 5.Milne TE, Rogers JR, Kinnear EM, Martin HV, Lazzarini PA, Quinton TR, et al. Developing an evidence-based clinical pathway for the assessment, diagnosis and management of acute Charcot neuro-arthropathy: A systematic review. J Foot Ankle Res 2013;6:30. [Google Scholar | PubMed]
- 6.O’Loughlin A, Kellegher E, McCusker C, Canavan R. Diabetic charcot neuroarthropathy: Prevalence, demographics and outcome in a regional referral centre. Ir J Med Sci 2017;186:151-6. [Google Scholar | PubMed]
- 7.Salini D, Harish K, Minnie P, Sundaram KR, Arun B, Sandya CJ, et al. Prevalence of Charcot arthropathy in type 2 diabetes patients aged over 50 years with severe peripheral neuropathy: A retrospective study in a tertiary care South Indian hospital. Indian J Endocrinol Metab 2018;22:107-11. [Google Scholar | PubMed]
- 8.Fauzi AA, Chung TY, Latif LA. Risk factors of diabetic foot Charcot arthropathy: A case-control study at a Malaysian tertiary care centre. Singapore Med J 2016;57:198-203. [Google Scholar | PubMed]
- 9.Papanas N, Maltezos E. Etiology, pathophysiology and classifications of the diabetic Charcot foot. Diabet Foot Ankle 2013;4:20872. [Google Scholar | PubMed]
- 10.Korzon-Burakowska A, Jakobkiewicz-Banecka J, Fiedosiuk A, Petrova N, Koblik T, Gabig-Ciminska M, et al. Osteoprotegerin gene polymorphism in diabetic Charcot neuroarthropathy. Diabet Med 2012;29:771-5. [Google Scholar | PubMed]
- 11.Bruhn-Olszewska B, Korzon-Burakowska A, Wegrzyn G, Jakobkiewicz-Banecka J. Prevalence of polymorphisms in OPG, RANKL and RANK as potential markers for Charcot arthropathy development. Sci Rep 2017;7:501. [Google Scholar | PubMed]
- 12.Pitocco D, Zelano G, Gioffre G, Di Stasio E, Zaccardi F, Martini F, et al. Association between osteoprotegerin G1181C and T245G polymorphisms and diabetic Charcot neuroarthropathy: A case-control study. Diabetes Care 2009;32:1694-7. [Google Scholar | PubMed]
- 13.Rosskopf AB, Loupatatzis C, Pfirrmann CW, Boni T, Berli MC. The Charcot foot: A pictorial review. Insights Imaging 2019;10:77. [Google Scholar | PubMed]
- 14.Trieb K. The Charcot foot: Pathophysiology, diagnosis and classification. Bone Joint J 2016;98-B:1155-9. [Google Scholar | PubMed]
- 15.Lovy A, Dowdell J, Keswani A, Koehler S, Kim J, Wienfeld S, et al. Nonoperative versus operative treatment of displaced ankle fractures in diabetics. Foot Ankle Int 2017;28:255-60. [Google Scholar | PubMed]
- 16.Manway JM, Blazek CD, Burns PR. Special considerations in the management of diabetic ankle fractures. Curr Rev Musculoskelet Med 2018;11:445-55. [Google Scholar | PubMed]
- 17.Durgia H, Sahoo J, Kamalanathan S, Palui R, Sridharan K, Raj H. Role of bisphosphonates in the management of acute Charcot foot. World J Diabetes 2018;9:115-26. [Google Scholar | PubMed]
- 18.Bartl C, Imhoff A, Bartl R. Treatment of bone marrow edema syndrome with intravenous ibandronate. Arch Orthop Trauma Surg 2012;132:1781-8. [Google Scholar | PubMed]
- 19.Petrova NL, Edmonds ME. Medical management of Charcot arthropathy. Diabetes Obes Metab 2012;15:193-7. [Google Scholar | PubMed]
- 20.Jude EB, Selby PL, Burgess J, Lilleystone P, Mawer EB, Page SR, et al. Bisphosphonates in the treatment of Charcot neuroarthropathy: A double-blind randomised controlled trial. Diabetologia 2001;44:2032-7. [Google Scholar | PubMed]
- 21.Pitocco D, Ruotolo V, Caputo S, Mancini L, Collina CM, Manto A, et al. Six-month treatment with Alendronate in acute Charcot neuroarthropathy: A randomized controlled trial. Diabetes Care 2005;28:1214-5. [Google Scholar | PubMed]
- 22.Richard JL, Almasri M, Schuldiner S. Treatment of acute Charcot foot with bisphosphonates: A systematic review of the literature. Diabetologia 2012;55:1258-64. [Google Scholar | PubMed]
- 23.Ito A, Yoshimura M. Mechanisms of the analgesic effect of calcitonin on chronic pain by alteration of receptor or channel expression. Mol Pain 2017;13:1-11. [Google Scholar | PubMed]
- 24.Gouveri E, Papanas N. Charcot osteoarthropathy in diabetes: A brief review with an emphasis on clinical practice. World J Diabetes 2011;2:59-65. [Google Scholar | PubMed]
- 25.Yousaf S, Dawe EJ, Saleh A, Gill IR, Wee A. The acute Charcot foot in diabetics: Diagnosis and management. EFORT Open Rev 2018;3:5668-73. [Google Scholar | PubMed]
- 26.Pinzur MS, Gil J, Belmares J. Treatment of osteomyelitis in Charcot foot with single-stage resection of infection, correction of deformity and maintenance with ring fixation. Foot Ankle Int 2012;33:1069-74. [Google Scholar | PubMed]
- 27.Lavery LA, Asmstrong DG, Wunderlich RP, Mohler J, Wendel CS, Lipsky BA. Risk factors for foot infections in individuals with diabetes. Diabetes Care 2006;29:1288-93. [Google Scholar | PubMed]
- 28.Berli M, Vlachopoulos L, Leupi S, Boni T, Baltin C. Treatment of Charcot neuroarthropathy and osteomyelitis of the same foot: A retrospective cohort study. BMC Musculoskeletal Disord 2017;18:460. [Google Scholar | PubMed]
- 29.Vopat ML, Nentwig MJ, Chong AC, Agan JL, Shields NN, Yang SY. Initial diagnosis and management for acute Charcot neuroarthropathy. Kans J Med 2018;11:114-9. [Google Scholar | PubMed]
- 30.Smith WB, Moore CA. A proposed treatment algorithm for midfoot Charcot arthropathy. Foot Ankle Spec 2012;5:60-4. [Google Scholar | PubMed]
- 31.Sommer TC, Lee TH. Charcot foot: The diagnostic dilemma. Am Fam Physician 2001;64:1591-8. [Google Scholar | PubMed]
- 32.Game FL, Catlow R, Jones GR, Edmonds ME, Jude EB, Rayman G, et al. Audit of acute Charcot’s disease in the UK: The CDUK study. Diabetologia 2012;55:32-5. [Google Scholar | PubMed]
- 33.Donegan R, Sumpio B, Blume PA. Charcot foot and ankle with osteomyelitis. Diabet Foot Ankle 2013;4:21361. [Google Scholar | PubMed]
- 34.Hartemann-Heurtier A, Senneville E. Diabetic foot osteomyelitis. Diabet Metab 2008;34:87-95. [Google Scholar | PubMed]
- 35.Gratwohl V, Jentzsch T, Schoni M, Kaiser D, Berli MC, Boni T, et al. Long-term follow-up of conservative treatment of Charcot feet. Arch Orthop Trauma Surg 2022;142:2553-66. [Google Scholar | PubMed]
- 36.Caputo GM, Ulbrecht J, Cavanagh PR, Juliano P. The Charcot foot in diabetes: Six key points. Am Fam Physician 1998;57:2705-10. [Google Scholar | PubMed]








