One must always consider the significance of sagittal imbalance and the potential role for operative orthopedic intervention or even correction, particularly when managing such a young patient cohort with such a severely morbid disease process such as osteoporosis.
Dr. Martin S Davey, Department of Trauma and Orthopaedics, University Hospital Galway, Galway, Ireland, E-mail: martindavey@rcsi.ie
Introduction: Although rare in incidence, pregnancy-induced osteoporosis (PIO)-associated OVCFs represent a significant cause of morbidity for the young, peri-partum female population.
Case Report: We present the case of a 27-year-old nulliparous lady who suffered seven osteoporosis vertebral compression fractures (OVCFs) with associated sagittal imbalance, the challenges posed to the attending physician or surgeon in treating this rare condition, as well as an in-depth discussion of previous literature reported on pregnancy-induced osteoporosis (PLIO) to date. Although rare in incidence, PLIO-associated OVCFs represent a significant cause of morbidity for the young, peripartum female.
Conclusion: This case demonstrates how multiple PLIO-associated OVCFs may be managed successfully, with careful consideration of sagittal imbalance, using a combination of medical and non-operative orthopedic therapies at medium-term follow-up.
Keywords: Pregnancy, Osteoporosis, Vertebral Compression Fractures, PLIO, Management
While osteoporosis is a common metabolic bone disease which occurs in both genders, its incidence tends to be much higher in the female population, particularly in the postmenopausal cohort [1]. Although the vast majority of female cases of osteoporosis focus on the postmenopausal patient, recent literature has demonstrated that peripartum females can be susceptible to pregnancy-induced osteoporosis (PIO) [2]. More commonly, potential breastfeeding-associated osteoporosis is widely acknowledged in the literature; however, its effect on actual bone loss has been deemed transitionory, with many physiological and homeostatic mechanisms at play postpartum [3]. A full-term neonate requires approximately thirty grams of calcium during pregnancy, with the majority of calcium transfer from mother to fetus occurring in the third trimester [4]. Although pregnancy has been described as a physiologic absorptive hypercalciuric state, rarely, more severe changes with decreases in bone mineral density have been described [5]. PIO conditions, such as post-pregnancy spinal osteoporosis and transient osteoporosis of the hip (TOH), are thought to be the result of the failure of the normal metabolic adaptations of pregnancy albeit without any definitive understanding of the underlying pathology [6,7]. Although rare, PIO can affect not only the development of associated vertebral fractures but also potentially alter sagittal imbalance, both of which can have extremely disabling consequences in young female patients [8]. Furthermore, the rarity of such presentations means that optimal management of such fractures presents a unique challenge to the attending surgeon, with treatment focused on potential prevention and/or correction of resulting deformities. Therefore, the primary aim of this study sought to report our findings in a young patient who presented with PIO.
A healthy nulliparous (G1+0) 27-year-old female with no previous medical history presented to the emergency department early in her second trimester after developing severe acute onset lower back pain, in the absence of any history of trauma. On clinical examination, it was reported that she displayed point tenderness at the level of the thoracolumbar junction, and difficulty ambulating secondary to pain, with no evidence of any radiculopathy or lower limb neurological deficits. Plain film radiographs were performed in anteroposterior and lateral views of her lumbar spine alone demonstrating compression fractures of her 1st (30%) and 2nd (50%) lumbar vertebrae (Fig. 1).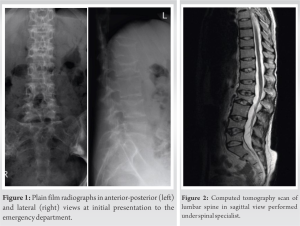
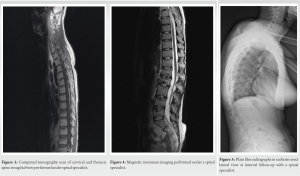
She was subsequently referred to the fracture clinic at 1-week postpartum, where she was initially treated in a soft thoracolumbar spinal orthosis (TLSO) with an associated referral to our institution’s board-certified, fellowship-trained orthopedic spinal surgeon at 3-week postpartum. Although the various management options were discussed at length with our patient, she was keen to progress with conservative management of her numerous fractures. No risk factor or predisposing condition was identified, and the patient’s dietary assessment reflected adequate calcium intake. Serological laboratory investigations demonstrated low 25 (OH) Vitamin D levels (26 nmol: Normal range >50). However, the interval plain film radiographs performed at the spinal clinic 2 weeks later showed the progression of her lumbar spine into kyphosis and positive sagittal balance. Thereafter, a computed tomography scan revealed biconcave compression fractures of the T9, T11, T12 (all with 20% loss of height), L1 (30%), L2 (50%), L4 (30%), and L5 (40%) vertebrae (Fig. 2). The middle and posterior columns remained intact at each vertebral level [9]. There was no evidence of any involvement of the posterior elements on magnetic resonance imaging and short-TI Inversion recovery sequence confirmed the fractures to be recent. She had a low pelvic incidence, low lumbar lordosis, and a compensatory hypokyphosis of the thoracic spine (Fig. 3 and 4). A summary of the sagittal balance parameters is shown in Table 1.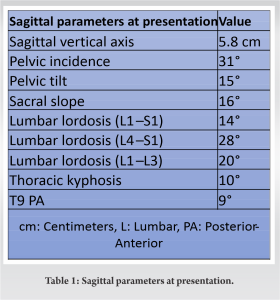
A dual-energy X-ray absorptiometry (DEXA) scan was subsequently performed diagnosing gross osteoporosis in the lumbar spine with T-scores ranging from −4 to −4.4 (converted to Z-scores of −3.9 to −4.3), notably more severe than measurements at the femoral head. The results of the DEXA scan are further illustrated in Table 2.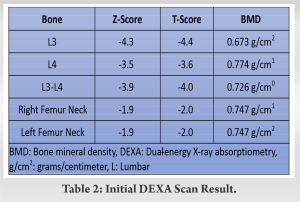
Treatment with bracing was continued and in consultation with the bone health service (which in our institution includes specialist endocrinology and rheumatology services), further actions implemented with the aim of halting bone loss included cessation of breastfeeding. In an effort to combat potential exacerbation of her now-known severe osteoporotic condition, the patient was thereafter commenced on a 24 month course of 20 mg of teriparatide once daily subcutaneously and the addition of calcium carbonate 500 mg and Vitamin D3 800 international units combined once daily orally. All potential benefits and risks of such treatment were outlined in full before commencement. At 4-month follow-up, she reported improvement in back pain with no myelopathic or radicular features, with no neurological deficits reported globally on examination. In addition, her TLSO brace was weaned to long-distance mobilization only, with a subsequent referral for physiotherapy to strengthen core stability exercises as well as tolerated. Prominent kyphosis at the thoracolumbar junction remained evident on clinical examination and imaging. The low lumbar lordosis and compensatory low thoracic kyphosis both remained stable through the follow-up period. Further improvement in back pain and increased mobility were reported at 7-month follow-up and repeat imaging showed that lumbar lordosis, thoracic kyphosis, and sagittal parameters were unchanged from the previous. Serological laboratory lives of 25 (OH) Vitamin D had normalized (76 nmol/L) at this point. No surgical intervention was required throughout the patient’s clinical course. The patient was successfully discharged from the service at 18-month follow-up following counseling regarding potential long-term sequelae and the option for review as needed, with a prescription of 6 monthly injections of denosumab. At 6-year follow-up (Fig. 5), her latest DEXA scan reported moderate osteopenia, with a T-Score of −1.5. At the final follow-up, the patient was asked for permission that data related to this case would be converted into anonymized form with the intention of publication. The patient subsequently provided informed consent in written form for this use.
Epidemiology
While the incidence of PIO is debated in the literature, however, consensus exists that spinal involvement represents a rare sequelae, with a reported incidence of approximately 0.4/100,000 [10,11]. PIO classically presents in primigravid women in the first 3-month postpartum [12], with acute onset back pain, but up to 40% of those affected will at the time or retrospectively describe symptoms during the third trimester [13]. Furthermore, neither lactation nor multiple pregnancies have been shown to be associated with subsequent osteoporosis [14], nor is there a reported association between the duration of lactation and fracture risk [15]. However, studies demonstrate that a proportion of patients with PIO may have a genetic predisposition to low peak bone mass, which may contribute to their increased risk of fracture during pregnancy [16], while the presence of premenopausal fractures has been shown to increase the patient’s risk of postmenopausal fracture by 35% [17]. Furthermore, a combination of congenital and acquired risk factors has been identified for PIO, namely, the presence of low body weight, family history, Vitamin D deficiency, and smoking [18]. A retrospective study performed over a 20-year period by O’Sullivan et al. [19] found that in their 11 patient series, over 80% of patients had at least one risk factor for osteoporosis, despite their young age and pre-menopausal status.
TOH
Although uncommon, TOH represents a more common clinical phenomenon to PIO which is noted to be more prevalent in the male population [20]. Many known risk factors for TOH match those pre-disposing individuals to osteoporosis, including low serum Vitamin-D levels, steroid usage, and low circulating phosphate and calcium levels [21,22]. First reported by Curtiss and Kincaid in 1959, pregnancy was noted to represent a risk factor for TOH in cases whereby the young female patient is affected, with significant demineralization of four proximal femurs [23]. With respect to both PIO and TOH, previous literature has found that although the pregnant lady is at increased risk of reduced bone density, any associated bone loss is believed to be physiologically reversible in the postpartum period [24]. Furthermore, previous series have found satisfactory clinical outcomes for both osteoporotic vertebral compression fractures (OVCFs) and TOH, with little postpartum morbidity reported in the long term [19,20].
Sagittal imbalance
The sagittal alignment, or “front to back balance,” of a normal spine is directly related to physiological spinal alignment by the muscular forces [25]. Therefore, sagittal balance is dependent on the positional relationship between the thoracic curve, lumbar curve, sacrum, and the pelvis. A normal sagittal vertical axis is reflected in the plumb line lying within 3 cm of the sacral promontory (anteriorly or posteriorly); however, this balance may be altered positively (plumb line lies >3 cm anteriorly) or negatively (plumb line lies >3 cm posteriorly [26]. Such imbalances may be due to intrinsic alignment issues or changes in alignment due to any number of musculoskeletal pathologies, such as PIO in the case of the lady previously discussed in this case.
Management
The management of osteoporosis in the general population is well established, with specific involvement of appropriate investigations for any underlying secondary cause, including Vitamin D deficiency, coeliac disease, anorexia nervosa, mastocytosis, and hyperparathyroidism and hyperthyroidism [27]. However, as a paucity of prospective literature exists for cases of PIO, optimal treatment strategies for such cases remain controversial [28]. Recommendations for initial avoidance of strenuous activities, appropriate analgesia and physiotherapy as well as appropriate re-mineralization with calcium and Vitamin D supplementation tend to be incorporated into medical management in the majority of cases [29]. Although discrepancies in opinion exist among obstetricians and pediatricians globally, logical cessation of lactation in cases of PIO reduces the deleterious effect on the bodies’ calcium stores, with these initial steps alone have been shown to result in complete resolution of symptoms at 4 months [30]. Regarding further pharmacological treatment, there is a far weaker consensus. In all cases, a major concern that exists when treating gravid women evolves around potential teratogenicity or implications fetal development [31,32]. Both bisphosphonates and teriparatide fall into the United States Federal Drug Administration’s Category C, theoretically rendering them contra-indicated during pregnancy unless there is a strong clinical argument for their use [33]. Stumpf et al. previously demonstrated that in the absence of an underlying cause, bone mineral density will return toward previous levels post-pregnancy granted that suitable osteoanabolic therapy is utilized [34]. In addition, consideration as to whether bisphosphonates or teriparatide should be used in these cases is debated; teriparatide has previously been reported that result in a greater positive effect on bone mineral density when compared to bisphosphonates in PIO [35], with lower reported pain scores than those treated with percutaneous kyphoplasty at short-term follow-up [36].
Non-operative orthopedic management
The use of bracing as an acute non-operative treatment for OVCFs is well established, but it remains largely opinion based, with a lack of underpinning evidence which is majorly based on their application in the management of acute, traumatic burst fractures [37]. In their meta-analysis of four randomized control trials, Jin and Lee suggested that bracing plays a role in significantly improving patient-reported pain scores and quality of life when compared to controls in patients with OVCFs [38], although two of the included trials were not supportive of bracing in such patients [39,40]. Theoretically, bracing decreases the load over the anterior spinal column by attempting to reduce forward flexion, therefore decreasing the painful contraction of erector spinae which occurs with the sagittal imbalance of multiple vertebral compression fractures [41]. Typically prescribed for a 3-month period, the use of bracing has previously been reported to prevent new or progression of old fractures at 12-month follow-up in young osteoporotic female patients suffering PIO, particularly in cases of thoracolumbar compression fractures when combined with supplementary medical and physical therapy [8,42-44]. Our patient was treated in a soft TLSO brace, although acknowledgment of the study Kim et al. [40] is warranted, who demonstrated that there were no significant differences in reported pain scores at 3-month follow-up between patients managed with a soft brace, a hard brace, or no brace at all. Although rare, our patient’s presentation with seven vertebral fractures is not unique. A previous study by Ofluoglu and Ofluoglu [45] reported the conservative management of a 30-year-old patient with PIO presenting with eight OVCFs in her spine, with acceptable results at 12-month follow-up. For their patient, the option of balloon kyphoplasty was considered with the patient; however, the concern of potential difficulty performing an osteotomy in future through a cement-filled vertebral body tipped the balance away from operative orthopedic management in her case. Osteotomy was held in reserve for our patient until well after the peripartum period in case potential symptomology or sagittal malalignment became unsatisfactory in our patient’s case in future. This option provided a potentially less demanding approach should operative intervention have been required, in comparison to a potential hypothetical situation in which a failed trial of kyphoplasty has altered native anatomy. Furthermore, our patient has reported satisfactory outcomes at 6-year follow-up, with occasional mild lumber back pain on exertion. Having been successfully discharged from our service, she is continuously been administered therapeutic denosumab on a bi-annual basis which is followed up in the community. Her latest DEXA scan reported a moderate osteopenia, with a T-Score of −1.5 (Z-score of −1.4).
Although rare in incidence, PIO-associated OVCFs represent a significant cause of morbidity for the young, peripartum female population. The lady in our case is an example of how multiple PIO-associated OVCFs may be managed successfully with a combination of medical and non-operative orthopedic therapies with medium-term follow-up. However, one must always consider the significance of sagittal imbalance and the potential role of operative orthopedic intervention or even correction, particularly when managing such a young patient cohort with such a severely morbid disease process such as osteoporosis.
The lady in our case is an example of how multiple PIO-associated OVCFs may be managed successfully with a combination of medical and non-operative orthopedic therapies with medium-term follow-up; however, the significance of sagittal imbalance must be a consideration when electing either management option.
References
- 1.Black DM, Rosen CJ. Clinical practice. Postmenopausal osteoporosis. N Engl J Med 2016;374:254-62. [Google Scholar | PubMed]
- 2.Salari P, Abdollahi M. The influence of pregnancy and lactation on maternal bone health: A systematic review. J Family Reprod Health 2014;8:135-48. [Google Scholar | PubMed]
- 3.Grizzo FM, Alarcão AC, Dell’Agnolo CM, Pedroso RB, Santos TS, Vissoci JR, et al. How does women’s bone health recover after lactation? A systematic review and meta-analysis. Osteoporos Int 2020;31:413-27. [Google Scholar | PubMed]
- 4.Givens MH, Macy IG. The chemical composition of the human fetus. J Biol Chem 1933;102:7-17. [Google Scholar | PubMed]
- 5.Gertner JM, Coustan DR, Kliger AS, Mallette LE, Ravin N, Broadus AE. Pregnancy as state of physiologic absorptive hypercalciuria. Am J Med 1986;81:451-6. [Google Scholar | PubMed]
- 6.Asadipooya K, Graves L, Greene LW. Transient osteoporosis of the hip: Review of the literature. Osteoporos Int 2017;28:1805-16. [Google Scholar | PubMed]
- 7.Laroche M, Talibart M, Cormier C, Roux C, Guggenbuhl P, Degboe Y. Pregnancy-related fractures: A retrospective study of a French cohort of 52 patients and review of the literature. Osteoporos Int 2017;28:3135-42. [Google Scholar | PubMed]
- 8.Bonacker J, Janousek M, Kröber M. Pregnancy-associated osteoporosis with eight fractures in the vertebral column treated with kyphoplasty and bracing: A case report. Arch Orthop Trauma Surg 2014;134:173-9. [Google Scholar | PubMed]
- 9.Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine (Phila Pa 1976) 1983;8:817-31. [Google Scholar | PubMed]
- 10.Smith R, Athanasou NA, Ostlere SJ, Vipond SE. Pregnancy-associated osteoporosis. QJM 1995;88:865-78. [Google Scholar | PubMed]
- 11.Hellmeyer L, Kühnert M, Ziller V, Schmidt S, Hadji P. The use of i. v. bisphosphonate in pregnancy-associated osteoporosis--case study. Exp Clin Endocrinol Diabetes 2007;115:139-42. [Google Scholar | PubMed]
- 12.Rizzoli R, Bonjour JP. Pregnancy-associated osteoporosis. Lancet 1996;347:1274-6. [Google Scholar | PubMed]
- 13.Khovidhunkit W, Epstein S. Osteoporosis in pregnancy. Osteoporos Int 1996;6:345-54. [Google Scholar | PubMed]
- 14.Paton LM, Alexander JL, Nowson CA, Margerison C, Frame MG, Kaymakci B, et al. Pregnancy and lactation have no long-term deleterious effect on measures of bone mineral in healthy women: A twin study. Am J Clin Nutr 2003;77:707-14. [Google Scholar | PubMed]
- 15.Michaëlsson K, Baron JA, Farahmand BY, Ljunghall S. Influence of parity and lactation on hip fracture risk. Am J Epidemiol 2001;153:1166-72. [Google Scholar | PubMed]
- 16.Peris P, Guañabens N, Monegal A, Pons F, Martínez de Osaba MJ, Ros I, et al. Pregnancy associated osteoporosis: The familial effect. Clin Exp Rheumatol 2002;20:697-700. [Google Scholar | PubMed]
- 17.Hosmer WD, Genant HK, Browner WS. Fractures before menopause: A red flag for physicians. Osteoporos Int 2002;13:337-41. [Google Scholar | PubMed]
- 18.Sowers M. Pregnancy and lactation as risk factors for subsequent bone loss and osteoporosis. J Bone Miner Res 1996;11:1052-60. [Google Scholar | PubMed]
- 19.O’Sullivan SM, Grey AB, Singh R, Reid IR. Bisphosphonates in pregnancy and lactation-associated osteoporosis. Osteoporos Int 2006;17:1008-12. [Google Scholar | PubMed]
- 20.Hadidy AM, Al Ryalat NT, Hadidi ST, Tarawneh ES, Hadidi MT, Samara OA, et al. Male transient hip osteoporosis: Are physicians at a higher risk? Arch Osteoporos 2009;4:41-5. [Google Scholar | PubMed]
- 21.Diwanji SR, Cho YJ, Xin ZF, Yoon TR. Conservative treatment for transient osteoporosis of the hip in middle-aged women. Singapore Med J 2008;49:e17-21. [Google Scholar | PubMed]
- 22.Bashaireh KM, Aldarwish FM, Al-Omari AA, Albashaireh MA, Hajjat M, Al-Ebbini MA, et al. Transient osteoporosis of the hip: Risk and therapy. Open Access Rheumatol 2020;12:1-8. [Google Scholar | PubMed]
- 23.Curtiss PH Jr., Kincaid WE. Transitory demineralization of the hip in pregnancy. A report of three cases. J Bone Joint Surg Am 1959;41-A:1327-33. [Google Scholar | PubMed]
- 24.Ensom MH, Liu PY, Stephenson MD. Effect of pregnancy on bone mineral density in healthy women. Obstet Gynecol Surv 2002;57:99-111. [Google Scholar | PubMed]
- 25.Le Huec JC, Thompson W, Mohsinaly Y, Barrey C, Faundez A. Sagittal balance of the spine. Eur Spine J 2019;28:1889-905. [Google Scholar | PubMed]
- 26.Iyer S, Lenke LG, Nemani VM, Fu M, Shifflett GD, Albert TJ, et al. Variations in occipitocervical and cervicothoracic alignment parameters based on age: A prospective study of asymptomatic volunteers using full-body radiographs. Spine (Phila Pa 1976) 2016;41:1837-44. [Google Scholar | PubMed]
- 27.Kanis JA, Cooper C, Rizzoli R, Reginster JY. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 2019;30:3-44. [Google Scholar | PubMed]
- 28.Campos-Obando N, Oei L, Hoefsloot LH, Kiewiet RM, Klaver CC, Simon ME, et al. Osteoporotic vertebral fractures during pregnancy: Be aware of a potential underlying genetic cause. J Clin Endocrinol Metab 2014;99:1107-11. [Google Scholar | PubMed]
- 29.Zhang M, Chen P, Li B, Du J, Pan T, Chen J. Approach to the patient with pregnancy and lactation-associated osteoporosis: A case report and a review of the literature. Medicine (Baltimore) 2017;96:e8671. [Google Scholar | PubMed]
- 30.Polat SB, Evranos B, Aydin C, Cuhaci N, Ersoy R, Cakir B. Effective treatment of severe pregnancy and lactation-related osteoporosis with teriparatide: Case report and review of the literature. Gynecol Endocrinol 2015;31:522-5. [Google Scholar | PubMed]
- 31.Sokal A, Elefant E, Leturcq T, Beghin D, Mariette X, Seror R. Pregnancy and newborn outcomes after exposure to bisphosphonates: A case-control study. Osteoporos Int 2019;30:221-9. [Google Scholar | PubMed]
- 32.Choe EY, Song JE, Park KH, Seok H, Lee EJ, Lim SK, et al. Effect of teriparatide on pregnancy and lactation-associated osteoporosis with multiple vertebral fractures. J Bone Miner Metab 2012;30:596-601. [Google Scholar | PubMed]
- 33.FDA classification of drugs for teratogenic risk. Teratology Society Public Affairs Committee. Teratology 1994;49:446-7. [Google Scholar | PubMed]
- 34.Stumpf UC, Kurth AA, Windolf J, Fassbender WJ. Pregnancy-associated osteoporosis: An underestimated and underdiagnosed severe disease. A review of two cases in short- and long-term follow-up. Adv Med Sci 2007;52:94-7. [Google Scholar | PubMed]
- 35.Hellmeyer L, Boekhoff J, Hadji P. Treatment with teriparatide in a patient with pregnancy-associated osteoporosis. Gynecol Endocrinol 2010;26:725-8. [Google Scholar | PubMed]
- 36.Kong M, Zhou C, Zhu K, Zhang Y, Song M, Zhang H, et al. 12-Month teriparatide treatment reduces new vertebral compression fractures incidence and back pain and improves quality of life after percutaneous kyphoplasty in osteoporotic women. Clin Interv Aging 2019;14:1693-703. [Google Scholar | PubMed]
- 37.Caitriona C, Mark MG, Elaine H, Claire G, Michelle F, Persson UM, et al. Management of hospitalised osteoporotic vertebral fractures. Arch Osteoporos 2020;15:14. [Google Scholar | PubMed]
- 38.Jin YZ, Lee JH. Effect of brace to osteoporotic vertebral fracture: A meta-analysis. J Korean Med Sci 2016;31:1641-9. [Google Scholar | PubMed]
- 39.Li M, Law SW, Cheng J, Kee HM, Wong MS. A comparison study on the efficacy of SpinoMed® and soft lumbar orthosis for osteoporotic vertebral fracture. Prosthet Orthot Int 2015;39:270-6. [Google Scholar | PubMed]
- 40.Kim HJ, Yi JM, Cho HG, Chang BS, Lee CK, Kim JH, et al. Comparative study of the treatment outcomes of osteoporotic compression fractures without neurologic injury using a rigid brace, a soft brace, and no brace: A prospective randomized controlled non-inferiority trial. J Bone Joint Surg Am 2014;96:1959-66. [Google Scholar | PubMed]
- 41.Longo UG, Loppini M, Denaro L, Maffulli N, Denaro V. Conservative management of patients with an osteoporotic vertebral fracture: A review of the literature. J Bone Joint Surg Br 2012;94:152-7. [Google Scholar | PubMed]
- 42.Pola E, Colangelo D, Nasto LA, Pambianco V, Autore G, Formica VM, et al. Pregnancy-associated osteoporosis (PAO) with multiple vertebral fragility fractures: Diagnosis and treatment in a young primigravid woman. J Biol Regul Homeost Agents 2016;30:153-8. [Google Scholar | PubMed]
- 43.Takahashi N, Arai I, Kayama S, Ichiji K, Fukuda H, Handa J, et al. Four-year follow-up of pregnancy-associated osteoporosis: A case report. Fukushima J Med Sci 2014;60:175-80. [Google Scholar | PubMed]
- 44.Iwamoto J, Sato Y, Uzawa M, Matsumoto H. Five-year follow-up of a woman with pregnancy and lactation-associated osteoporosis and vertebral fractures. Ther Clin Risk Manag 2012;8:195-9. [Google Scholar | PubMed]
- 45.Ofluoglu O, Ofluoglu D. A case report: Pregnancy-induced severe osteoporosis with eight vertebral fractures. Rheumatol Int 2008;29:197-201. [Google Scholar | PubMed]