Fibrous dysplasia of the proximal radius, if symptomatic, can be treated by curettage and filling the defect with a non-vascularized fibular strut graft, along with intramedullary nailing for stabilization and early resumption of ROM.
Dr. Aashiket Sable, Department of Orthopaedics, Bharat Ratna Dr. Babasaheb Ambedkar Municipal General Hospital, Mumbai, Maharashtra, India. E-mail: aashiketsable67@gmail.com
Introduction: Fibrous dysplasia (FD) is a skeletal developmental abnormality commonly affecting the ribs, femur, tibia, skull, pelvis, spine, and shoulder. FD of the proximal radius is extremely rare and very few cases have been reported. In addition, monostotic lesions of FD in the upper extremity go unnoticed as they are usually asymptomatic. Symptomatic lesions warrant surgical intervention. Here, we present a rare case of FD of the proximal radius treated with curettage and non-vascularized fibular cortical strut graft with intramedullary elastic nailing. We believe that this is the first report in the literature wherein this treatment modality has been undertaken.
Case Report: A 27-year-old woman presented with excruciating pain and swelling in her right elbow for 4 weeks, with no inciting event or trauma leading to the pain. Plain radiographs revealed a well-circumscribed radiolucent lesion in the proximal radius with cortical thinning at the metaphysis and a rim of epiphyseal bone. Clinically, the patient had restricted supination (50°) and limited elbow range of motion (ROM) (20–130°), mostly because of the pain but had full pronation. With these radiographic and clinical features, FD and giant cell tumor were kept as differential diagnoses and surgical treatment was planned. The lesion was excised leaving the normal epiphysis of the radius intact and samples were sent for histopathological examination. A non-vascularized fibular cortical strut graft was harvested from the same side and was fluted into the radial shaft. Final stabilization was done using a 2.5 mm intramedullary elastic nail. The arm was immobilized in an above-elbow slab. Histopathology confirmed our diagnosis of FD. The slab was removed after 6 weeks, and a gentle ROM was started in the form of active-assisted ROM. At the end of 1 year, complete union and almost full ROM were achieved and the patient was completely pain-free.
Conclusion: Non-vascularized fibular strut grafting with intramedullary nailing provides a comparatively quicker, cost-effective way of treating this lesion with a minimum insult of the bony cortex and quicker rehabilitation.
Keywords: Fibrous dysplasia, proximal radius, strut graft, intramedullary nailing.
Fibrous dysplasia (FD) is a non-inherited, skeletal developmental abnormality commonly affecting the ribs, femur, tibia, skull, pelvis, spine, and shoulder [1-4]. In the long bones, it mostly affects the diaphysis and epiphyseal lesions are rare [5]. First introduced by Lichtenstein in 1938, it frequently presents in adolescents and young adults and accounts for 5–7% of the huge spectrum of benign bone tumors [1,6,7]. In this condition, normal cancellous woven bone is replaced by weak, immature woven bone and a dense fibrotic stroma containing a disorganized matrix of bony trabecular spicules [8], making it abnormally fragile and more prone to fracture. It may be monostotic (70–80%) or polyostotic [5]. Very few cases of FD have been reported in the proximal radius, as in the upper extremity it mostly affects the proximal humerus [9-11]. Being mostly asymptomatic, pain, restriction of movements, deformity, and chances of pathologic fracture are the chief complaints in the symptomatic population and surgical treatment is mainly palliative [12]. Radiographically, these lesions are known to have a “ground glass appearance” as they are well-circumscribed, hazy, and radiolucent, sometimes compromising the structural integrity of the bone causing bowing of weight-bearing bones like the femur (“shepherd’s crook” deformity) [13-15]. Indications of surgery include non-union, progressive deformity, or persistent pain [2,3,4]. Smaller lesions, especially of the monostotic variety can be treated conservatively with the risk that these lesions heal but are susceptible to repeat fracture because of the dysplastic nature of the bony callus [16-18]. Surgical treatment options include curettage and fibular cortical strut grafting, with the grafts being either cortical, cancellous, or vascularized [16-18]. With this report, we present a case of FD of the proximal radius in a 27-year-old female treated with fibular cortical strut graft and intramedullary nailing.
A 27-year-old right-hand dominant woman presented to the outpatient department with excruciating pain and swelling in her right elbow for 4 weeks, which was insidious in onset with no inciting event or trauma leading to the pain.
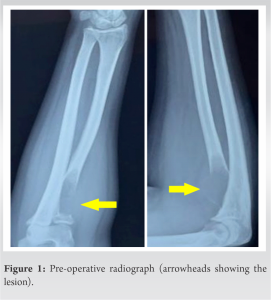
Plain radiographs revealed a well-circumscribed radiolucent lesion in the proximal radius with cortical thinning at the metaphysis and a rim of epiphyseal bone (Fig. 1). Magnetic resonance imaging scan of the elbow showed a well-defined centric lytic lesion in the meta-diaphyseal area involving the radial tuberosity and proximal third of the radius measuring 2.7 × 1.5 × 5 cm, located about 7 mm from the articular surface (Fig. 2). Clinically, the patient had restricted supination (50°) and limited elbow range of motion (ROM) (20–130°), mostly because of the pain but had full pronation. The results of endocrine and laboratory studies were normal.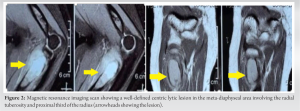
As the symptoms warranted surgical intervention, the decision was made to proceed with curettage and ORIF of the lesion. The proximal radius was explored using Henry’s approach, the intermuscular interval being flexor carpi radialis and brachioradialis while the inter-nervous plane being the median and radial nerves. The lesion was excised leaving the normal epiphysis of the radius intact. Samples were sent for histopathological examination, keeping FD and a giant cell tumor (GCT) as our chief differential diagnoses. A non-vascularized fibular cortical strut graft was harvested from the same side, keeping its length 1 cm more than the excised bone to avoid shortening. The graft was fluted and wedged in the radial shaft and final stabilization was done using a 2.5 mm intramedullary elastic nail. Immediate post-operative radiographs were obtained, and the arm was immobilized in an above-elbow slab with the elbow in 90° flexion and the forearm in full supination.
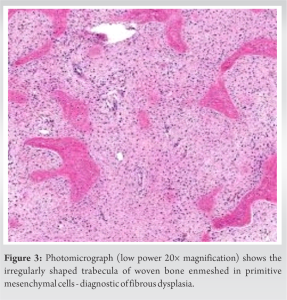
The histopathology report confirmed our diagnosis of FD (Fig. 3). The slab was removed after 6 weeks and a gentle ROM was started in the form of active assisted forearm pronation and supination, as well as elbow flexion and extension. Passive ROM was started once the pain subsided at 8 weeks and strengthening exercises were gradually started at 3 months post-surgery using resistance bands, as per tolerance. Regular follow-ups were done every month for the first 3 months and then every 3 months thereafter till 1 year. At the end of 1 year, the complete union was achieved (Fig. 4) and the patient was pain-free, with full pronation, 80° supination (comparable to the left forearm), and almost full elbow ROM (10–140°).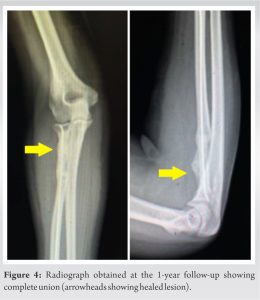
FD may either present as a single monostotic lesion or as a polyostotic variety. Polyostotic FD is associated with abnormal cutaneous lesions and certain endocrinal disorders, characteristic of McCune Albright syndrome or Mazabraud syndrome [3,4,19]. This leads to an early recognition of these lesions. On the other hand, monostotic lesions are difficult to identify as they present silently and are only diagnosed accidentally when radiographs of the involved extremity are taken due to other reasons [8,20]. As in our case, the lesion was undiagnosed till the symptoms appeared and thus, a radiograph was taken which raised our suspicion (Fig. 1). Yet, we kept the GCT as our differential apart from FD due to its location near the joint and the cortical expansion on plain radiographs. FD is found in the upper extremity, most commonly in the proximal humerus [8]. Very few cases of FD are found in and around the elbow [5,9,20]. Campanacci has reported only 9 lesions around the elbow, 2 of which were found in the distal humerus and 7 in the proximal radius [6]. The majority of these upper extremity lesions is asymptomatic and can be conserved. The symptomatic lesions have been successfully treated with curettage and bone grafting (cortical autografts/allografts or vascularized bone grafts) with or without ORIF [16,18]. One of the prime challenges faced by the surgeon in such cases is recurrence. Such lesions when treated with cancellous bone grafts tend to recur as these grafts heal by creeping substitution relying on the local healing response as part of the remodeling process. Therefore, immature woven bone reforms in the defect leading to mechanically weak bone like the one present before the excision [5,21]. Thus, a cortical strut graft is preferred in such cases. The use of fibular cortical strut grafts has been proven in literature [22,23]. It is known that vascularized autologous cortical bone does not weaken as it does not undergo resorption, which makes it a superior graft as compared to its non-vascularized counterpart [22,23]. Kumta et al. reported eight patients who were treated with a similar graft for FD of the upper limb [10]. On the contrary, George et al., treated 17 patients with FD lesions of the proximal femur with a non-vascularized fibular strut graft, which was easier to harvest in a shorter operative time [24]. We used a similar strategy for the elbow using a non-vascularized graft. Patankar et al. successfully treated a similar lesion of the proximal radius using a non-vascularized fibular cortical strut graft without ORIF, after which the patient was immobilized in a long arm cast for 6 months, and gradual ROM was started later [9]. In our case, we similarly jam-packed a fibular strut graft but stabilized the construct of an elastic nail because of which we could start mobilizing the elbow and forearm as early as 6 weeks after surgery, thus, avoiding complications due to prolonged immobilization such as stiffness and muscle atrophy. Kokkalis et al. treated two patients with similar lesions. The first was a 39-year-old female with a painful lesion of the proximal radius and the second was a 33-year-old female with a proximal radius lesion along with a pathological fracture in the area. Both were treated with curettage. In both cases, allografts were used, and the constructs were stabilized using non-locking plates [5]. We used an intramedullary elastic nail which is a low-profile, low-cost implant as compared to the bulkier plates. Another advantage of using a nail was that we could cut the tourniquet time short, therefore, avoiding the complications that occur due to prolonged tourniquet time as the primary surgery itself is time-consuming.
FD lesions around the elbow are rare. A high index of suspicion is needed to diagnose these lesions early, to prevent aggravation of symptoms, and to avoid complications such as pathological fractures. The surgeon should be flexible because the size, location of the lesion, and quality of bone are variable in each patient. Surgery should be offered when symptomatic. In our case presented here, we believe we could provide a comparatively quicker, cost-effective way of treating this lesion with a minimum insult of the bony cortex and quicker rehabilitation. The patient was able to regain her ROM and was completely pain-free at the end of 1 year.
FD of the proximal radius is a rare condition. If symptomatic, it can be treated by curettage and filling the defect with a non-vascularized fibular strut graft, along with intramedullary nailing for stabilization and early resumption of ROM.
References
- 1.Resnick D. Fibrous dysplasia. In: editor. Diagnosis of Bone and Joint Disorders. 4th ed. Philadelphia, PA: WB Saunders; 2002. p. 4825-43. [Google Scholar | PubMed]
- 2.Kumar R, Madewell JE, Lindell MM, Swischuk LE. Fibrous lesions of bones. Radiographics 1990;10:237-56. [Google Scholar | PubMed]
- 3.Parekh SG, Donthineni-Rao R, Ricchetti E, Lackman RD. Fibrous dysplasia. J Am Acad Orthop Surg 2004;12:305-13. [Google Scholar | PubMed]
- 4.Wilner D. Fibrous dysplasia of bone. In: Radiology of Bone Tumors and Allied Disorders. Philadelphia, PA: Saunders; 1982. p. 1443-80. [Google Scholar | PubMed]
- 5.Kokkalis ZT, Jain S, Sotereanos DG. Fibrous dysplasia around the elbow. J Shoulder Elbow Surg 2010;19:e6-11. [Google Scholar | PubMed]
- 6.Campanacci M. Bone and Soft Tissue Tumors: Clinical Features, Imaging, Pathology and Treatment. 2nd ed. New York: Springer; 1999. p. 435-62. [Google Scholar | PubMed]
- 7.Chapurlat RD, Meunier PJ. Fibrous dysplasia of bone. Baillieres Best Pract Res Clin Rheumatol 2000;14:385-98. [Google Scholar | PubMed]
- 8.Harris WH, Dudley HR Jr., Barry RJ. The natural history of fibrous dysplasia. An orthopaedic, pathological, and roentgenographic study. J Bone Joint Surg Am 1962;44-A:207-33. [Google Scholar | PubMed]
- 9.Patankar H, Patankar S, Tandon N, Bairy A. Fibrous dysplasia of radius bone-excision and fibula graft: A case report. J Orthop Case Rep 2022;12:31-4. [Google Scholar | PubMed]
- 10.Kumta SM, Leung PC, Griffith JF, Kew J, Chow LT. Vascularised bone grafting for fibrous dysplasia of the upper limb. J Bone Joint Surg Br 2000;82:409-12. [Google Scholar | PubMed]
- 11.Matsuya S, Hatori M, Hosaka M, Ito K, Dohi O, Endo M, et al. Operative treatment by external fixation for polyostotic fibrous dysplasia in the elbow joint. A case report. Ups J Med Sci 2006;111:269-74. [Google Scholar | PubMed]
- 12.DiCaprio MR, Enneking WF. Fibrous dysplasia. Pathophysiology, evaluation, and treatment. J Bone Joint Surg Am 2005;87:1848-64. [Google Scholar | PubMed]
- 13.Fitzpatrick KA, Taljanovic MS, Speer DP, Graham AR, Jacobson JA, Barnes GR, et al. Imaging findings of fibrous dysplasia with histopathologic and intraoperative correlation. AJR Am J Roentgenol 2004;182:1389-98. [Google Scholar | PubMed]
- 14.Guille JT, Kumar SJ, MacEwen GD. Fibrous dysplasia of the proximal part of the femur. Long-term results of curettage and bone-grafting and mechanical realignment. J Bone Joint Surg Am 1998;80:648-58. [Google Scholar | PubMed]
- 15.Kransdorf MJ, Moser RP Jr., Gilkey FW. Fibrous dysplasia. Radiographics 1990;10:519-37. [Google Scholar | PubMed]
- 16.Demiralp B, Ozturk C, Ozturan K, Sanisoglu YS, Cicek IE, Erler K. Prophylactic intramedullary nailing in monostotic fibrous dysplasia. Acta Orthop Belg 2008;74:386-90. [Google Scholar | PubMed]
- 17.Enneking WF. Clinical Musculoskeletal Pathology. 3rd ed. Gainesville, FL: University of Florida; 1990. p. 266-7. [Google Scholar | PubMed]
- 18.Keijser LC, Van Tienen TG, Schreuder HW, Lemmens JA, Pruszczynski M, Veth RP. Fibrous dysplasia of bone: Management and outcome of 20 cases. J Surg Oncol 2001;76:157-66. [Google Scholar | PubMed]
- 19.Jhala DN, Eltoum I, Carroll AJ, Lopez-Ben R, Lopez-Terrada D, Rao PH, et al. Osteosarcoma in a patient with McCune-Albright syndrome and Mazabraud’s syndrome: A case report emphasizing the cytological and cytogenetic findings. Hum Pathol 2003;34:1354-7. [Google Scholar | PubMed]
- 20.Henry A. Monostotic fibrous dysplasia. J Bone Joint Surg Br 1969;51:300-6. [Google Scholar | PubMed]
- 21.Burchardt H, Enneking WF. Transplantation of bone. Surg Clin North Am 1978;58:403-27. [Google Scholar | PubMed]
- 22.Finkemeier CG. Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am 2002;84:454-64. [Google Scholar | PubMed]
- 23.Dell PC, Burchardt H, Glowczewskie FP Jr. A roentgenographic, biomechanical, and histological evaluation of vascularized and non-vascularized segmental fibular canine autografts. J Bone Joint Surg Am 1985;67:105-12. [Google Scholar | PubMed]
- 24.George B, Abudu A, Grimer RJ, Carter SR, Tillman RM. The treatment of benign lesions of the proximal femur with non-vascularised autologous fibular strut grafts. J Bone Joint Surg Br 2008;90:648-51. [Google Scholar | PubMed]










