Collaborative and multidisciplinary efforts are essential for precise diagnosis and prompt integrated treatment of chronic recurrent multifocal osteomyelitis and any potential complications.
Dr. Josip Lovaković, Department of Surgery, University Hospital Centre Zagreb, Kišpatićeva 12, 10000, Zagreb, Croatia. E-mail: jopa.lovakovic@gmail.com
Introduction: Chronic recurrent multifocal osteomyelitis (CRMO) is a rare, skeletal, autoinflammatory disorder that predominantly affects young females. In this case-based review, we present a girl with progressive CRMO affecting the spine and causing spinal cord compression that required two surgical interventions.
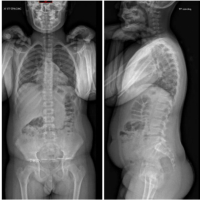
Case Report: A 10-year-old girl was presented to the pediatric rheumatologist because of diffuse back pain radiating to her legs, a waddling gait, and sensitivity to palpation in the caudal cervical vertebral region for the past several months. During the extensive multidisciplinary evaluation, spine magnetic resonance imaging (MRI) revealed C7 vertebral body collapse requiring reconstructive surgery. The pathohistological findings of the vertebral body samples indicated chronic inflammation, whereas the microbiological analysis was negative. Because CRMO was suspected, indomethacin therapy was started with slow regression of initial symptoms and further regular controls by surgeon and pediatric rheumatologist. Six months after the first operation, without any symptoms, the patient underwent regular control X-ray of the cervical spine, which revealed C6 vertebral body collapse. Soon after the second reconstructive surgery, she presented with subacute thoracic pain due to Th7 vertebral collapse, as verified by repeated MRI. No other skeletal lesions were detected. Finally, the tumor necrosis factor inhibitor adalimumab was initiated, which resulted in the slow resolution of pain and the lack of new symptoms.
Conclusion: Spine involvement in CRMO can lead to serious deformities and even life-threatening fractures. Effective multidisciplinary cooperation involving experienced surgeons, radiologists, pathologists, and rheumatologists is crucial for accurate diagnosis and timely combined management of CRMO and possible complications.
Keywords: Chronic recurrent multifocal osteomyelitis, spine, surgery, case-based review.
Chronic recurrent multifocal osteomyelitis (CRMO) is the most severe type of chronic nonbacterial osteomyelitis (CNO) and is a non-infectious, auto-inflammatory skeletal disorder that usually affects children and adolescents. With the significant increase in the incidence of CRMO during the past two decades, females are twice as affected as males, and the average age at disease onset is 9–10 years. Pathogenesis is strongly associated with the dysregulation of cytokine expression and various inflammatory diseases, primarily psoriasis and inflammatory bowel disease. The most prominent clinical features are local bone pain and swelling [1]. Disease-predilection sites are the metaphyses of long bones, mandible, clavicle, and pelvis, while one-third of all patients have some types of spinal column involvement, most commonly in the thoracic region [2,3]. The spinal presentation of pathological fractures or vertebral deformities ranges from asymptomatic to functional neurological deficit associated with spinal cord compression. An extremely small number of CRMO patients with spine involvement are surgically treated [4]. We present a rare case of aggressive CRMO causing multiple vertebral body collapse accompanied by spinal cord compression that required two reconstructive surgeries, combined with a concise literature review of CRMO cases affecting the vertebral column that required surgical intervention.
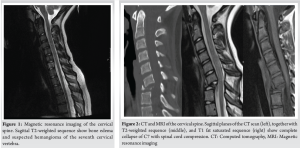
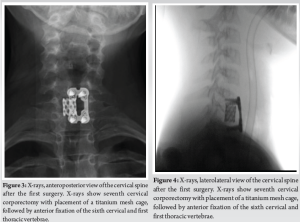
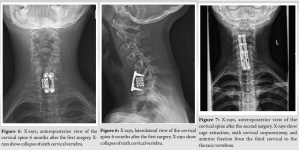
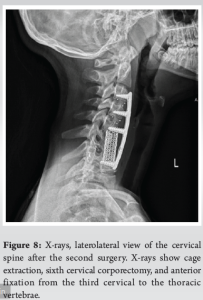
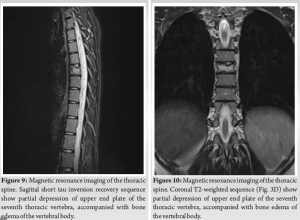
A 10-year-old female patient initially reported to her local hospital with complaints of morning back pain accompanied by gait and stiffness for the last several months. In the beginning, the symptoms were mild and diminished after a few hours; however, they slowly progressed in intensity and duration. Before these symptoms, she was a healthy child who was actively engaged in gymnastics, but due to progressive pain, she stopped training. She was afebrile all the time, and she did not have night sweats, lymphadenopathy, or any skin changes. On the first pediatric rheumatologist appointment, slight tenderness to palpation over the spinous process of the seventh cervical (C7) vertebra was noted. There were no skin lesions. The neurological status, including muscle strength of the lower limbs, was normal. Laboratory evaluation, including assessment of inflammatory parameters, fecal occult blood tests, and stool S-100 results were normal/negative. Human leukocyte antigen B27 was negative. Electromyography of the lover limbs was normal. Spinal X-rays were normal, while initial magnetic resonance imaging (MRI) of the brain, spine, and pelvis revealed suspected hemangioma of the C7 vertebra (Fig. 1), together with bilateral acetabular bone edema. Skeletal scintigraphy revealed an inhomogeneous distribution without focal pathological accumulation of Tc-99m diphosphonate. A couple of weeks later, severe progression of the initial symptoms combined with mild elevation of ESR and CRP (30 mm/h and 3.2 mg/L, respectively) was noted, while second MRI of the whole spine, along with computed tomography (CT), highlighted prolapse of the C7 vertebra and compression of the spinal cord (Fig. 2). The patient was urgently fitted with a Philadelphia cervical collar, and a few days later, she underwent C7 corporectomy with placement of a titanium mesh cage, followed by anterior fixation of the sixth cervical (C6) and first thoracic vertebrae (Th1) (Fig. 3 and 4.). Postoperative histopathological samples revealed clear signs of chronic inflammation, whereas microbiology evaluation including Mycobacterial culture and a quantiferon test were negative. Due to the patient’s anamnesis, clinical presentation, and results of extensive evaluation, a diagnosis of CRMO was suspected, and indomethacin + sulfasalazine therapy was started. During the further regular controls by surgeon and pediatric rheumatologist, she was pain-free, with limited terminal cervical spine movements. Six months after the first operative procedure, during the regular check-up, the control X-ray revealed collapse of the C6 vertebra (Figs. 5 and 6). The patient underwent a second surgical procedure: Cage extraction, C6 corporectomy, and anterior fixation from the third cervical to the Th1 vertebra (Fig. 7 and 8). Soon after the second reconstructive surgery, she presented with subacute thoracic pain due to Th7 vertebral collapse without spinal cord compression, as verified by repeated MRI (Fig. 9 and 10.). No other skeletal lesions were detected. With a Z-score of zero, bone densitometry revealed normal mineral bone density. The patient was fitted with an extension brace, and combination of indomethacin and sulfasalazine was replaced with the biological disease-modifying antirheumatic drug adalimumab combined with methotrexate, which resulted in slow resolution of pain and lack of new symptoms for the last few months.
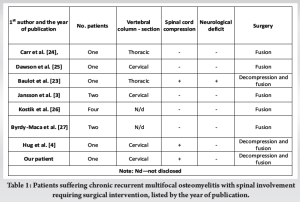
Since first described in 1972 [5] and actually given a formal name a few years later [6], the term “CRMO” implies multiple sterile, painful, skeletal lesions with recurrent periods of exacerbation and remission in most cases, predominantly occurring during childhood. Synovitis, acne, pustulosis, hyperostosis, osteitis syndrome, and another variant of CNO occur more frequently in adults and are associated with a higher incidence of skin lesions [1]. Based on the widely accepted diagnostic criteria of Bristol [7] and Jansson [3], our patient almost fulfilled all the requirements for CRMO. Similar to our patient, CRMO typically leads to localized symptoms in affected areas, considerably diminishing overall physical and daily functions. On the other hand, our patient showed no signs of skin lesions, fever, weight loss, or fatigue, which are other possible symptoms but are not essential for diagnosis [8]. In the last few decades, CRMO cases have been suspected to be underreported, with an incidence of 0.4/100,000 children per year [9]. Because of the pronounced similarities in presentation, patients were usually misdiagnosed with bacterial osteomyelitis and were occasionally treated with antibiotics [10]. The difference in incidence between these two aforementioned entities is significantly smaller, if present, according to Schnabel et al. [11]. In addition to pathological fracture, vertebral lesions can cause partial or complete collapse of the affected vertebra, eventually manifesting as vertebra plana or deformity in terms of kyphosis and scoliosis [12]. Several factors contribute to the delay in CRMO diagnosis of approximately 2 years [13]. First, non-specific local symptoms, in conjunction with systemic features, can present together with single or multiple bone lesions [14]. Second, CRMO remains the primary diagnosis of exclusion, with a broad differential diagnosis. Third, laboratory results are frequently vague, with possible slight elevations in inflammatory parameters. In addition, radiological evaluation can depict lytic or sclerotic bone lesions depending on the disease stage; however, it can also be negative or uninformative for different abnormalities [1]. In our case, a definitive CRMO diagnosis was established almost 12 months after the initial symptoms, after the fracture and first C7 surgical reconstructive procedure. Despite the significant time reduction until diagnosis in our case compared with previous reports [13], there is still a glaring delay, mostly due to the normal initial findings in the laboratory and other diagnostic evaluations combined with the uninformative first spine MRI finding and suspicion of C7 hemangioma. Because up to 25% of all CRMO patients will experience some degree of spinal lesion [15], and approximately 17% will suffer a vertebral fracture [16], with even higher specific complication incidence in more recent studies [17], even shorter diagnostic delay than average can play a significant role in morbidity, as in our patient. In cases of CNO suspicion, bone scintigraphy was the main imaging method in the diagnostic process, but due to its drawbacks, such as the accumulation of radionuclides in the growth plates and exposure to radiation, it was replaced with whole-body (WB) MRI. In addition to being more sensitive and highlighting inflammation of surrounding soft tissues, WB-MRI is extremely important when searching for “silent” lesions, which are mostly present in the vertebral column [18]. In our case, bone scintigraphy impressed as most sensitive diagnostic tool at the time of initial evaluation. It revealed an inhomogeneous distribution without focal pathological accumulation of Tc-99m, but possibly omitting already present, still “silent” spine lesions. Although not mandatory for diagnosis, bone biopsy, which was also performed in our patient, is usually performed for the final confirmation of CNO diagnosis due to factors mentioned earlier regarding clinical presentation and the diagnostic process [7]. In patients with CNO, conservative treatment is always indicated, independent of the presence of spinal lesions. First-line medicament therapy includes non-steroidal anti-inflammatory drugs (NSAIDs), followed by second-line therapy encompassing conventional, biological, and targeted DMARDs [19] and/or bisphosphonates [11]. According to Schnabel et al., NSAIDs are the first-line treatment in cases without vertebral column involvement because almost half of patients with spinal lesions experience relapse within 2 years while on NSAIDs. In more demanding cases, bisphosphonates and other DMARDs were associated with a greater decrease in symptoms and higher remission rates [11]. Compared with the consensus treatment plans [1] for refractory CRMO cases with active spine lesions, our patients’ treatment followed a similar pattern with slight delay. After the initial administration of indomethacin postoperatively, sulfasalazine was added. Subsequently, with disease progression and after the second surgical procedure, adalimumab was introduced as second-line therapy in the consensus plan. Several algorithms have been developed with the goal of accurately classifying and providing treatment guidelines for fracture and deformity management affecting the subaxial cervical and thoracolumbar spine. After the 3-column model, which is based on fracture morphology and radiological assessment [20], the thoracolumbar injury classification and severity score and subaxial cervical spine injury classification and severity score both include fracture morphology as well as the neurological status of the patient and the integrity of the posterior ligamentous complex [21,22]. A specificity with pediatric patients that must be considered when deciding on treatment options is skeletal growth. Conservative treatment entails appropriate analgesia, rest, and bracing, depending on the affected vertebral column area. Orthotics attenuate pain and provide external support, and they can be used to prolong surgical procedures or even as a definitive treatment method until the resolution of the lesion. On the other hand, neurological deficit, severe and progressive deformity, and unsatisfactory results in conservative treatment are indications for surgical treatment [4]. To the best of our knowledge, there are only a few cases of CRMO with spinal involvement in children who underwent surgical intervention (Table 1), and none of them had recurrent/new lesions that required two surgeries, as was the case in our patient. Most patients underwent fusion due to progressive deformity and increased spinal cord compression. In addition to our patient, only two patients had spinal cord compression [4,23], while one of them also had a progressive neurological deficit [23]. Both patients underwent decompression and fusion.
Although CRMO is relatively rare, it should be considered in the differential diagnosis of local bone pain and unspecific systemic symptoms. In progressive and demanding cases, such as our case, it is especially important to diagnose the condition with as little delay as possible to prevent possible complications. Despite the best efforts to avoid surgical intervention in children, spine surgeons should play a key role in the early, multidisciplinary evaluation and treatment of patients with vertebral lesions to prevent subsequent neurological deficits due to fractures and deformities.
While surgical procedures in children should be approached with caution, prompt diagnosis of CRMO, and the initiation of both medical and, if necessary, invasive treatments are essential for the patient’s benefit.
References
- 1.Zhao Y, Ferguson PJ. Chronic nonbacterial osteomyelitis and chronic recurrent multifocal osteomyelitis in children. Pediatr Clin North Am 2018;65:783-800. [Google Scholar | PubMed]
- 2.Roderick MR, Sen ES, Ramanan AV. Chronic recurrent multifocal osteomyelitis in children and adults: Current understanding and areas for development. Rheumatology (Oxford) 2018;57:41-8. [Google Scholar | PubMed]
- 3.Jansson A, Renner ED, Ramser J, Mayer A, Haban M, Meindl A, et al. Classification of non-bacterial osteitis: Retrospective study of clinical, immunological and genetic aspects in 89 patients. Rheumatology (Oxford) 2007;46:154-60. [Google Scholar | PubMed]
- 4.Hug NF, Purger DA, Li D, Rinsky L, Hong DS. Neurosurgical management of vertebral lesions in pediatric chronic recurrent multifocal osteomyelitis: Patient series. J Neurosurg Case Lessons 2023;5:CASE22179. [Google Scholar | PubMed]
- 5.Giedion A, Holthusen W, Masel LF, Vischer D. Subacute and chronic “symmetrical” osteomyelitis. Ann Radiol (Paris) 1972;15:329-42. [Google Scholar | PubMed]
- 6.Probst FP, Björksten B, Gustavson KH. Radiological aspect of chronic recurrent multifocal osteomyelitis. Ann Radiol (Paris) 1978;21:115-25. [Google Scholar | PubMed]
- 7.Roderick MR, Shah R, Rogers V, Finn A, Ramanan AV. Chronic recurrent multifocal osteomyelitis (CRMO) - advancing the diagnosis. Pediatr Rheumatol Online J 2016;14:47. [Google Scholar | PubMed]
- 8.Buch K, Thuesen AC, Brøns C, Schwarz P. Chronic non-bacterial osteomyelitis: A review. Calcif Tissue Int 2019;104:544-53. [Google Scholar | PubMed]
- 9.Jansson AF, Grote V, ESPED Study Group. Nonbacterial osteitis in children: Data of a German incidence surveillance study. Acta Paediatr 2011;100:1150-7. [Google Scholar | PubMed]
- 10.Silier CC, Greschik J, Gesell S, Grote V, Jansson AF. Chronic non-bacterial osteitis from the patient perspective: A health services research through data collected from patient conferences. BMJ Open 2017;7:e017599. [Google Scholar | PubMed]
- 11.Schnabel A, Range U, Hahn G, Siepmann T, Berner R, Hedrich CM. Unexpectedly high incidences of chronic non-bacterial as compared to bacterial osteomyelitis in children. Rheumatol Int 2016;36:1737-45. [Google Scholar | PubMed]
- 12.Yasin S, Sato TS, Ferguson P. Not all benign: Disease course, complications, and sequalae of chronic recurrent multifocal osteomyelitis in children. Curr Opin Rheumatol 2022;34:255-61. [Google Scholar | PubMed]
- 13.Oliver M, Lee TC, Halpern-Felsher B, Murray E, Schwartz R, Zhao Y, et al. Disease burden and social impact of pediatric chronic nonbacterial osteomyelitis from the patient and family perspective. Pediatr Rheumatol Online J 2018;16:78. [Google Scholar | PubMed]
- 14.Zhao DY, McCann L, Hahn G, Hedrich CM. Chronic nonbacterial osteomyelitis (CNO) and chronic recurrent multifocal osteomyelitis (CRMO). J Transl Autoimmun 2021;4:100095. [Google Scholar | PubMed]
- 15.Hospach T, Langendoerfer M, Von Kalle T, Maier J, Dannecker GE. Spinal involvement in chronic recurrent multifocal osteomyelitis (CRMO) in childhood and effect of pamidronate. Eur J Pediatr 2010;169:1105-11. [Google Scholar | PubMed]
- 16.Wipff J, Costantino F, Lemelle I, Pajot C, Duquesne A, Lorrot M, et al. A large national cohort of French patients with chronic recurrent multifocal osteitis. Arthritis Rheumatol 2015;67:1128-37. [Google Scholar | PubMed]
- 17.O’Leary D, Wilson AG, MacDermott EJ, Lowry C, Killeen OG. Variability in phenotype and response to treatment in chronic nonbacterial osteomyelitis; the Irish experience of a national cohort. Pediatr Rheumatol 2021;19:45. [Google Scholar | PubMed]
- 18.Fritz J, Tzaribatchev N, Claussen CD, Carrino JA, Horger MS. Chronic recurrent multifocal osteomyelitis: Comparison of whole-body MR imaging with radiography and correlation with clinical and laboratory data. Radiology 2009;252:842-51. [Google Scholar | PubMed]
- 19.Benjamin O, Goyal A, Lappin SL. Disease-modifying antirheumatic drugs (DMARD). In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2024. [Google Scholar | PubMed]
- 20.Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine (Phila Pa 1976) 1983;8:817-31. [Google Scholar | PubMed]
- 21.Vaccaro AR, Lehman RA Jr., Hurlbert RJ, Anderson PA, Harris M, Hedlund R, et al. A new classification of thoracolumbar injuries: The importance of injury morphology, the integrity of the posterior ligamentous complex, and neurologic status. Spine (Phila Pa 1976) 2005;30:2325-33. [Google Scholar | PubMed]
- 22.Vaccaro AR, Hulbert RJ, Patel AA, Fisher C, Dvorak M, Lehman RA Jr., et al. The subaxial cervical spine injury classification system: A novel approach to recognize the importance of morphology, neurology, and integrity of the disco-ligamentous complex. Spine (Phila Pa 1976) 2007;32:2365-74. [Google Scholar | PubMed]
- 23.Baulot E, Bouillien D, Giroux EA, Grammont PM. Chronic recurrent multifocal osteomyelitis causing spinal cord compression. Eur Spine J 1998;7:340-3. [Google Scholar | PubMed]
- 24.Carr AJ, Cole WG, Roberton DM, Chow CW. Chronic multifocal osteomyelitis. J Bone Joint Surg Br 1993;75:582-91. [Google Scholar | PubMed]
- 25.Dawson JS, Webb JK, Preston BJ. Case report: Chronic recurrent multifocal osteomyelitis with magnetic resonance imaging. Clin Radiol 1994;49:133-6. [Google Scholar | PubMed]
- 26.Kostik MM, Kopchak OL, Maletin AS, Mushkin AY. The peculiarities and treatment outcomes of the spinal form of chronic non-bacterial osteomyelitis in children: A retrospective cohort study. Rheumatol Int 2020;40:97-105. [Google Scholar | PubMed]
- 27.Byrdy-Daca M, Duczkowski M, Sudoł-Szopińska I, Żelewska M, Piłat K, Daca F. Spinal involvement in patients with chronic non-bacterial osteomyelitis (CNO): An analysis of distinctive imaging features. J Clin Med 2023;12:7419. [Google Scholar | PubMed]