Knowledge of classical radiologic findings of CSA is key for prompt diagnosis and treatment of CSA. Long-term follow-up post-treatment of CSA is recommended given the complications of management and the progressive nature of CSA.
Mr. Jeff J Cherian, Baylor College of Medicine, Houston, Texas, United States. E-mail: jeff.cherian@bcm.edu
Introduction: Charcot spinal neuroarthropathy is a progressive destructive vertebral disease characterized by a loss of pain sensation and proprioception. Diagnosing this condition is particularly challenging because symptoms can appear at widely varying times and the neurological symptoms and imaging findings are non-specific. In contemporary cases, Charcot spine is often associated with chronic traumatic spinal cord injuries, in which the lack of proper sensation can hide the early signs of discovertebral destruction, making it even more difficult to promptly diagnose and treat.
Case Report: A 47-year-old female with a history of American Spinal Injury Association Grade A spinal cord injury presented with dysreflexia, postural changes, and imaging findings consistent with Charcot spine at the T11-T12 level. The patient underwent a successful posterior instrumented spinal fusion. Two years later, the patient presented with worsening pain and dysreflexia and was consequently diagnosed with a second Charcot spine at the L3-L4 level requiring an L3-pelvis fixation.
Conclusion: Charcot spinal arthropathy is a complex diagnosis of exclusion based on history and histopathologic and radiologic findings. This case adds to a very limited number of reports exploring the long-term outcomes of surgical management in Charcot spine and highlights the need for examining the relationship between surgical fusion and acceleration of vertebral joint destruction in hopes to help establish future management guidelines.
Keywords: Charcot spinal arthropathy, spinal neuroarthropathy, spinal cord injury, spinal fusion, pelvis fixation, pain, proprioception
Charcot spinal arthropathy (CSA), an uncommon subclass of progressive neuropathic joint diseases affecting the spine, was first reported in 1884 on a patient with spinal cord injury who had spondylolisthesis [1]. Further reported cases in the early 1900s indicated a significant association of spinal neuroarthropathy with tabes dorsalis [1]. Today, most cases of CSA have fewer tabetic etiologies – as cases of advanced syphilis have decreased – and are more frequently seen with spinal cord injuries (SCI) [2]. The proposed etiologies of CSA are broadly thought to be neurotraumatic and neurovascular. Causative SCIs generally result in a loss of proprioceptive and nociceptive innervation. Neurotraumatic theories propose that neuroprotective reflexes that would normally enable an even distribution of mechanical stresses over the joint through protective ligament stretch-muscle contraction are absent, resulting in repetitive microtrauma and progressive discovertebral destruction [3,4]. Progressive degeneration of the affected discovertebral level causes subsequent compression of adjacent nerve roots, resulting in pain and paresthesia along with autonomic dysreflexia [2]. Neurovascular theories propose that autonomic dysfunction results in hyperemia in subchondral bone, resulting in bone resorption and consequent microfractures, destruction, and joint instability [3,5]. CSA is often difficult to recognize because pre-existing neurologic deficits may obscure the initial clinical symptoms of CSA, which tend to be non-specific and vary in their time of onset from initial injury [6]. Furthermore, the classical imaging findings for CSA are non-specific and consequently result in a complex differential diagnosis also involving degenerative and infectious etiologies. As a result, CSA has a significant diagnostic delay, where the mean time between the onset of neurological impairment and a CSA diagnosis is 17.3 years ± 10.8 years [3]. General presentation of symptoms involves back pain, sitting imbalance, spinal postural changes – namely, kyphosis, autonomic dysreflexia (notably sweating and blood pressure variability), changes in spasticity, and noises associated with motion localized to the affected region [5,7]. The L2 and L3 vertebrae are, on average, the most affected by CSA [3]. To help identify the key imaging features of CSA and aid in its future diagnosis, we present the radiographic findings of a patient with CSA secondary to a traumatic SCI.
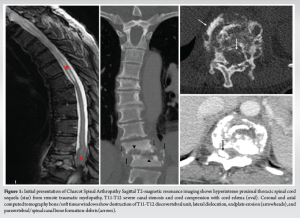
A 47-year-old female first presented with a history of C8 level American Spinal Injury Association Grade A spinal cord injury that occurred 22 years ago. The patient reported spasticity and moderate autonomic dysreflexia following the initial injury. At the first presentation, she exhibited popping noises in the mid back, kyphotic changes, and increased dysreflexia – involving increased episodes of bowel and bladder incontinence, flushing, and sweating with postural changes when sleeping, sitting, and transferring – that had worsened over several months. Imaging revealed destructive discovertebral changes in the T11-12 level and >1 cm lateral translation. The intervertebral disk was non-enhancing (Fig. 1). As the differential was concerning for CSA versus discitis-osteomyelitis on imaging, a biopsy was performed, ruling out evidence of infectious etiologies. Evaluation of histological samples exhibited fibrous tissue with bony remodeling but showed no evidence of tumors. Cultures were negative.
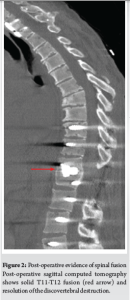
Surgery was performed as the primary therapeutic intervention, which included a T9-L3 vertebral level posterior spinal instrumented fusion (PSIF) and a T11-12 transforaminal interbody fusion, along with ligation of the right T11 nerve root (Fig. 2). After the surgery, the patient had significant improvement in her posture and her truncal kyphosis. Her nighttime sweats, postural change-dependent dysreflexia, and popping sounds resolved.
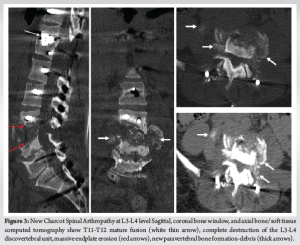
Two years later, the patient presented with malaise, recurrence of popping sounds, consistently increasing sympathetic dysreflexia with postural changes, abdominal pain, and worsening back pain. The patient exhibited increasing ankle instability with ankle eversion and greater spasticity in the lower extremities. Imaging revealed extension into the soft tissues with adjacent psoas involvement. A similar workup with biopsy was done to exclude infectious etiologies. Subsequent imaging revealed a recurrence of CSA at the L3-L4 level (Fig. 3). Surgery included L3-pelvis fixation with sacral-alar-iliac fixation (S2AI) screws, along with right L3 hemilaminectomy and facetectomy (Fig. 4). Following her second surgery, the patient reported significantly improved ankle positioning, resolution of sweating and malaise, and a mild decrease in spasticity. Follow-up imaging at 3 months showed improvement of soft tissue and psoas involvement, and imaging at 18 months showed complete resolution of their involvement.
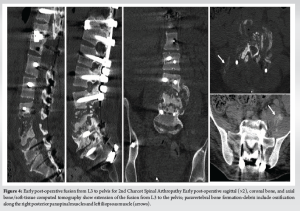
Diagnosis of CSA is difficult due to the variability in onset and the non-specific nature of its clinical symptoms. In addition, imaging findings of CSA mimic those of other spinal conditions such as discitis-osteomyelitis, hemodialysis-related spondyloarthropathy, spinal tuberculosis, degenerative spondylosis, and pseudoarthrosis at locations of previous spinal fusion [6]. Consequently, patients with CSA are often diagnosed late in the disease process. Although challenging, earlier recognition of CSA is vital in preventing further neurological deterioration and providing timely intervention [3,8]. Imaging is often key to the definitive diagnosis of CSA. In this case, our differential diagnosis included degenerative spondylosis, discitis-osteomyelitis, and CSA. Radiographic findings demonstrated the classic imaging features of CSA outlined in the 6Ds mnemonic: Density increase, Destruction of subchondral bone, Debris surrounding the discovertebral junction, Distention, Disorganization, and Dislocation [7].
Although spondylosis can also present with discovertebral architectural distortion, other typical degenerative findings on imaging like degenerative osteophytes or disk desiccation were absent. In addition, we were able to exclude spondylosis from the differential diagnosis because our patient presented with endplate erosion, whereas spondylosis typically preserves vertebral endplates. Accordingly, while discitis-osteomyelitis may also present with discovertebral destruction, endplate erosion, bone marrow edema, and vertebral collapse, the absence of paraspinal or epidural phlegmonous changes or fluid collections (abscesses) on imaging made discitis-osteomyelitis less likely. A subsequent biopsy and culture ruled out neoplastic and infectious etiologies. Clinical and imaging evidence of infection with negative serology, biopsy, and cultures should raise suspicion for culture-negative infections, which are observed in 37–50% of cases of discitis-osteomyelitis [9]. Factors including recent antibiotic therapy, infection by intracellular or fastidious organisms, inadequate pathogen yield, and technical sampling limitations can result in negative cultures. As such, molecular techniques such as polymerase chain reaction and metagenomic next-generation sequencing (mNGS) are increasingly useful for pathogen recognition. mNGS, a high-throughput sequencing technique, allows for sequencing of all microbial genetic material in a sample, avoiding the time-consuming process of selecting, culturing, and identifying microbes [10]. In a retrospective cross-sectional study of 114 patients with acute spinal infections, the sensitivity and specificity of mNGS were 95.5% and 31.4% respectively, with a positive percent agreement of 84.91% compared to 43.40% for conventional methods [11]. Patients with new-onset or worsening back pain, especially in the setting of elevated erythrocyte sedimentation rate (ESR) or C-reactive protein or infective endocarditis and bloodstream infections should be evaluated for infectious etiologies. Molecular techniques can be considered in those with negative cultures or serology and subsequent imaging-indicated biopsies that do not confirm a microbiologic diagnosis to enable timely antimicrobial treatment. Treatment options for CSA include conservative management, involving monitoring and non-surgical immobilization, or surgery – the more common alternative [2,7]. These management options should be critically weighed based on the specific patient presentation and goals of treatment. Current literature recommends circumferential arthrodesis as the preferred treatment as patients demonstrate increased functional status, decreases in pain, and improved sitting imbalance, as in this case [2,7]. However, there is a notable lack of research regarding the long-term outcomes of surgery and the relative effectiveness of other modalities [2]. Importantly, surgical complications include hardware failure, non-union, infection, and development of additional CSA adjacent to the surgical site, with a reoperation rate as high as 40% [2,6,12]. Patients who cannot tolerate surgical interventions or those with milder symptoms may be preferentially treated using conservative management, usually involving orthoses or braces to reduce mobility and slow progressive damage [2]. Conservative therapy may also involve adjunctive treatments for pain management with neurotropic painkillers and physical therapy and autonomic dysreflexia with anticholinergic drugs [2]. Considerations for surgical versus conservative management should include conversations on future functional status and pain management goals as well as the determination of tolerability of surgery. Delays in diagnosis can complicate surgical intervention as progressive destruction of the vertebral bodies and joint spaces reduce the likelihood of achieving a successful fusion. Furthermore, fusions – especially those involving the lumbar spine – form a lever arm resulting in increased force loads over inferior vertebral segments. In those patients that lack neuroprotective reflexes, this uneven load balancing may further advance vertebral degenerative processes leading to a new Charcot joint [13]. Post-PSIF surgery, our patient revealed a second incidence of CSA in their 2-year follow-up. The elected treatment was surgical extension of her initial fusion to the pelvis. Our case highlights the complications that can occur with CSA even after initial treatment and emphasizes the need for long-term clinical and radiological follow-up of CSA to track recurrence or emergence of existing/new Charcot joints. In addition, our case prompts the question regarding the nature of the relationship between surgical intervention and future developments of CSA. While current literature suggests that unbalanced, excessive biomechanical loads are a risk factor for the development of CSA, it remains unclear the extent to which surgical fusion and fixation contribute to this process [13]. More inquiry into this relationship is necessary to better understand and address post-operative complications. Further research is needed to help establish guidelines for diagnosis, treatment, and long-term management of CSA and reduce patient morbidity.
CSA, a rare progressive and destructive neuropathic joint disease, presents a significant diagnostic challenge. This case report illustrates the importance of patient history and histopathologic and radiologic findings in working through the differential diagnosis of CSA. It contributes to the limited research on CSA imaging findings to help aid in early diagnosis and timely prevention. Our case also demonstrates the difficulties of CSA treatment and stresses the need for a consensus in CSA management. CSA management guidelines should consider the short- and long-term benefits and risks of surgical versus conservative treatment and should emphasize regular, long-term clinical and radiologic follow-up after initial treatment.
Diagnosis of CSA can be challenging due to non-specific symptom presentation and imaging findings, variability in timing, and relative rarity of the condition. Management is complicated further as clear guidelines have not yet been proposed nor is there a clear consensus on medical interventions. Although surgical management is the mainstay treatment as it has shown short-term functional improvement, long-term outcomes of surgical evaluations, that is, reoperations, recurring CSA, and new-onset CSA, have not been elucidated. Long-term follow-up post-fusion is warranted to evaluate patients for success of fusion and potential progression of CSA.
References
- 1.Gupta R. A short history of neuropathic arthropathy. Clin Orthop Relat Res 1993;296:43-9. [Google Scholar | PubMed]
- 2.Urits I, Amgalan A, Israel J, Dugay C, Zhao A, Berger AA, et al. A comprehensive review of the treatment and management of Charcot spine. Ther Adv Musculoskelet Dis 2020;12:1-11. [Google Scholar | PubMed]
- 3.Barrey C, Massourides H, Cotton F, Perrin G, Rode G. Charcot spine: Two new case reports and a systematic review of 109 clinical cases from the literature. Ann Phys Rehabil Med 2010;53:200-20. [Google Scholar | PubMed]
- 4.Solinsky R, Donovan JM, Kirshblum SC. Charcot Spine following chronic spinal cord injury: An analysis of 201 published cases. Spinal Cord 2019;57:85-90. [Google Scholar | PubMed]
- 5.Farrugia PR, Bednar D, Oitment C. Charcot arthropathy of the spine. J Am Acad Orthop Surg 2022;30:e1358-65. [Google Scholar | PubMed]
- 6.Ledbetter LN, Salzman KL, Sanders RK, Shah LM. Spinal neuroarthropathy: Pathophysiology, clinical and imaging features, and differential diagnosis. Radiographics 2016;36:783-99. [Google Scholar | PubMed]
- 7.Lee D, Dahdaleh NS. Charcot spinal arthropathy. J Craniovertebr Junction Spine 2018;9:9-19. [Google Scholar | PubMed]
- 8.Lacout A, Lebreton C, Mompoint D, Mokhtari S, Vallée CA, Carlier RY. CT and MRI of spinal neuroarthropathy. Am J Roentgenol 2009;193:W505-14. [Google Scholar | PubMed]
- 9.Alavi SM, Petri F, Mahmoud OK, Igwilo-Alaneme R, El Zein S, Nassr AN, et al. Culture-negative native vertebral osteomyelitis: A narrative review of an underdescribed condition. J Clin Med 2024;13:5802. [Google Scholar | PubMed]
- 10.Gao Q, Liu Q, Zhang G, Lu Y, Li Y, Tang M, et al. Identification of pathogen composition in a Chinese population with iatrogenic and native vertebral osteomyelitis by using mNGS. Ann Med 2024;56:2337738. [Google Scholar | PubMed]
- 11.Wang C, Hu J, Gu Y, Wang X, Chen Y, Yuan W. Application of next-generation metagenomic sequencing in the diagnosis and treatment of acute spinal infections. Heliyon 2023;9:e13951. [Google Scholar | PubMed]
- 12.Del Arco Churruca A, Vázquez Bravo JC, Gómez Álvarez S, Muñoz Donat S, Jordá Llona M. Charcot arthropathy in the spine. Experience in our centre. About 13 cases. Review of the literature. Rev Esp Cir Ortop Traumatol (Engl Ed) 2021;65:461-8. [Google Scholar | PubMed]
- 13.Haus BM, Hsu AR, Yim ES, Meter JJ, Rinsky LA. Long-term follow-up of the surgical management of neuropathic arthropathy of the spine. Spine J 2010;10:e6-16. [Google Scholar | PubMed]