The gut microbiome profoundly influences orthopedic outcomes by regulating infection control, bone healing, and immune resilience, with microbiome-targeted therapies offering promising preventive and therapeutic strategies
Dr. Janki Sharan Bhadani, OPD No. 4, Department of Orthopaedics, Paras HMRI Hospital, Patna- 800014, Bihar, India. E-mail: jsbhadani@gmail.com
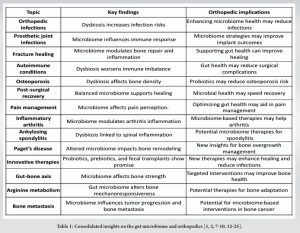
Advancements in microbiome-targeted therapies, including probiotics, prebiotics, and fecal microbial transplantation, offer exciting possibilities for orthopedic care. Probiotics, live beneficial microorganisms found in fermented foods, such as yogurt, kefir, sauerkraut, kimchi, and cheese, help maintain a healthy gut microbiome. Prebiotics and fiber-rich foods such as onions, garlic, and whole grains, nourish these bacteria, supporting their growth and activity. Together, these therapies regulate gut health, promote immune resilience, reduce infection risks, and accelerate healing – key factors in orthopedic outcomes [1,2]. The gut microbiome, a diverse ecosystem of microorganisms, plays a pivotal role in maintaining overall health [3]. Beyond digestion, it influences immune regulation, inflammation control, and musculoskeletal well-being [4]. Gut health significantly impacts orthopedic outcomes, including infection control, bone healing, and maintaining bone density. An imbalance in this ecosystem, known as dysbiosis, can compromise recovery and increase infection risks [5]. Supporting gut health through dietary modifications, probiotics, or prebiotics holds the potential to enhance patient outcomes [6].
Periprosthetic joint infections and fracture-related infections remain formidable challenges in orthopedic care. Biofilm formation on implants can protect bacteria from antibiotics and immune responses, complicating treatment [7]. Dysbiosis can further exacerbate these risks by allowing bacteria to enter the bloodstream and colonize surgical sites [8]. Maintaining a balanced gut microbiome can enhance the body’s immune defenses, limit bacterial migration, and promote implant longevity.
Bone repair is a finely regulated process involving inflammation, new bone formation, and remodeling [9]. A healthy gut microbiome plays an essential role in managing this process by moderating inflammation and promoting bone cell activity. Disruptions to the microbial ecosystem can lead to excessive inflammation and hinder the body’s ability to heal effectively [10]. Supporting microbial health may improve healing outcomes for patients prone to delayed recovery or complications.
Autoimmune diseases, such as rheumatoid arthritis, heighten susceptibility to infections due to weakened immune function [11]. Dysbiosis can further worsen immune imbalance, increasing the risk of bacterial migration to surgical sites. Ensuring gut health may help fortify immune resilience and reduce post-surgical complications for patients with these conditions [12].
The gut microbiome affects pain perception by influencing the production of neurotransmitters that regulate pain pathways. An imbalance in gut bacteria can lead to heightened pain sensitivity, posing challenges for managing chronic conditions such as osteoarthritis [13]. Optimizing gut health may offer an additional approach to complement traditional pain management strategies and reduce dependency on medications.
The gut microbiome plays a critical role in absorbing minerals vital for maintaining bone strength and density. Disruptions in microbial balance can impair this absorption process, contributing to conditions such as osteoporosis [14]. Emerging research suggests that specific probiotics may help enhance mineral absorption, providing a supportive therapy for improving bone health and reducing fracture risk [15].
The influence of the gut microbiome extends beyond local sites, playing a key role in systemic recovery following surgery. A balanced microbiome supports faster healing, reduced inflammation, and fewer post-operative complications. Conversely, microbial imbalances can delay recovery and increase the risk of infections [16]. Strategies to maintain microbial health in the perioperative period may improve surgical outcomes and accelerate rehabilitation.
Screening for microbial imbalances before surgery could allow for timely interventions, minimizing complications and improving outcomes. While some therapies remain experimental, they represent promising avenues for future developments in personalized orthopedic treatment strategies [17]. Recent research has revealed that gut microbial alterations, particularly those affecting arginine metabolism, play a significant role in influencing bone structural remodeling [18]. Mechanical loading is crucial for maintaining bone health, but its effectiveness is often hampered by high variability in bone mechanoreceptor activity influenced by gut microbes. Studies have shown that microbial depletion can profoundly influence this responsiveness, indicating a possible pathway for future therapeutic strategies. The gut-bone axis, a concept gaining increasing attention, connects the state of the microbiome with bone health [19]. This relationship opens up the potential for microbiome-targeted interventions, such as dietary changes or probiotics, to enhance bone strength and treat conditions such as osteoporosis and inflammatory arthritis. By understanding the microbial factors that influence bone metabolism, researchers are uncovering new mechanisms for improving bone health, offering hope for more effective treatments in the future. To summarize the diverse and critical roles of the gut microbiome in orthopedic practice, the following table highlights key conditions and their implications for patient care (Table 1).
The gut microbiome’s influence on infection, healing, and overall orthopedic outcomes presents an exciting area of exploration. For orthopedic surgeons and clinicians, understanding the gut microbiome’s role offers new preventive and therapeutic pathways, particularly in managing infection risks associated with implants and fractures. Future research may bring even more microbiome-targeted therapies, transforming orthopedic practices and improving patient care in ways that extend beyond traditional approaches. By integrating gut health into orthopedic treatment strategies, health-care providers can foster stronger, more resilient recovery for their patients, advancing the field toward more holistic and effective care.
References
- 1.Gulliver EL, Young RB, Chonwerawong M, D’Adamo GL, Thomason T, Widdop JT, et al. Review article: The future of microbiome-based therapeutics. Aliment Pharmacol Ther 2022;56:192-208. [Google Scholar | PubMed]
- 2.Leeuwendaal NK, Stanton C, O’Toole PW, Beresford TP. Fermented foods, health and the gut microbiome. Nutrients 2022;14:1527. [Google Scholar | PubMed]
- 3.Hou K, Wu ZX, Chen XY, Wang JQ, Zhang D, Xiao C, et al. Microbiota in health and diseases. Signal Transduct Target Ther 2022;7:135. [Google Scholar | PubMed]
- 4.Wu HJ, Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012;3:4-14. [Google Scholar | PubMed]
- 5.Hiltzik DM, Goodwin AM, Kurapaty SS, Inglis JE, Pagadala MS, Edelstein AI, et al. The role of the gut microbiome in orthopedic surgery-a narrative review. Curr Rev Musculoskelet Med 2024;17:37-46. [Google Scholar | PubMed]
- 6.Markowiak P, Śliżewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017;9:1021. [Google Scholar | PubMed]
- 7.Sharma S, Mohler J, Mahajan SD, Schwartz SA, Bruggemann L, Aalinkeel R. Microbial biofilm: A review on formation, infection, antibiotic resistance, control measures, and innovative treatment. [Published correction appears in Microorganisms 2024;12:1961]. Microorganisms 2023;11:1614. [Google Scholar | PubMed]
- 8.DeGruttola AK, Low D, Mizoguchi A, Mizoguchi E. Current understanding of dysbiosis in disease in human and animal models. Inflamm Bowel Dis 2016;22:1137-50. [Google Scholar | PubMed]
- 9.ElHawary H, Baradaran A, Abi-Rafeh J, Vorstenbosch J, Xu L, Efanov JI. Bone healing and inflammation: Principles of fracture and repair. Semin Plast Surg 2021;35:198-203. [Google Scholar | PubMed]
- 10.Maruyama M, Rhee C, Utsunomiya T, Zhang N, Ueno M, Yao Z, et al. Modulation of the inflammatory response and bone healing. Front Endocrinol (Lausanne) 2020;11:386. [Google Scholar | PubMed]








