Machine Learning-assisted preoperative planning for total hip arthroplasty demonstrates perfect clinical safety and substantially improved accuracy compared to traditional templating methods, establishing readiness for clinical decision-support integration.
Dr. Prashant Kumar Sharma, Department of Orthopaedics, Rama Medical College Hospital and Research Centre, Rama University, Kanpur, 209217, India. Email: drprashant.kg@gmail.com
Introduction: Machine learning (ML) applications in pre-operative templating for total hip arthroplasty (THA) require systematic validation through prediction-verification methodologies to establish clinical utility and safety.
Materials and Methods: We conducted a retrospective case series of 9 consecutive THA cases between March and September 2025. A hybrid ensemble ML system performed pre-operative templating using standardized radiographs with magnification correction. Predictions were systematically validated against actual intraoperative component selections through post-operative analysis. Primary outcomes included component-specific accuracy with descriptive statistics. Secondary outcomes included clinical safety and feasibility assessment.
Results: The ML system achieved 85.7% overall templating accuracy across all cases. Component-specific accuracies were stem type 100% (9/9), stem sizing 88.9% (8/9), cup sizing 66.7% (6/9), head sizing 54.4% (5/9), and material selection 95.6% (9/9). High templating accuracy (≥97.7%) was achieved in 44.4% of cases (4/9). The system demonstrated conversion risk prediction capability in one case. Clinical outcomes showed a 100% success rate with zero complications and no reoperations at a mean of 17.2 ± 8.1 weeks of follow-up.
Conclusion: ML-assisted pre-operative templating demonstrates feasibility for THA planning with encouraging accuracy results and excellent clinical safety. These findings support the potential for larger validation studies. The prediction-verification methodology provides a systematic framework for ML validation in orthopedic surgery.
Keywords: Machine learning, artificial intelligence, total hip arthroplasty, pre-operative planning, templating accuracy, post-operative validation.
Total hip arthroplasty (THA) represents one of the most successful orthopedic procedures, with over 450,000 procedures performed annually in the United States [1]. Accurate pre-operative templating remains crucial for optimal component selection, surgical planning, and implant longevity [2,3]. Traditional templating methods rely on surgeon experience with reported accuracy rates of 40–80% for component sizing [4,5].
Machine learning (ML) applications in orthopedic surgery have demonstrated potential for improving diagnostic accuracy and surgical outcomes [6,7]. Recent studies show promising results in automated radiographic analysis and implant sizing prediction [8,9]. However, most studies lack systematic validation methodologies comparing pre-operative ML predictions with actual post-operative outcomes [10].
The integration of AI in surgical planning requires robust validation frameworks ensuring clinical safety while demonstrating accuracy improvements over traditional methods [11,12]. Magnification correction protocols, essential for accurate templating, remain inconsistently applied across institutions [13,14].
This case series presents systematic validation of ML-assisted pre-operative templating through prediction-verification methodology. We developed a hybrid ensemble ML system and validated performance against actual surgical outcomes across 9 consecutive THA cases, emphasizing component-specific accuracy assessment and clinical safety validation.
Study design and ethical approval
This retrospective study analyzed consecutive primary THA cases performed at RAMA Medical College Hospital and Research Centre, between March and September 2025 following, institutional ethics committee approval. The study adhered to STROBE guidelines for case series reporting [15]. All patients provided written informed consent.
Patient population
Nine consecutive patients undergoing primary THA were included. Inclusion criteria were (1) primary osteoarthritis, inflammatory arthritis, or femoral neck fracture requiring THA; (2) high-quality pre-operative anteroposterior pelvis radiographs; (3) complete post-operative radiographic documentation; and (4) minimum 4 weeks follow-up. Exclusion criteria were (1) revision THA; (2) developmental hip dysplasia requiring specialized implants; and (3) inadequate radiographic quality.
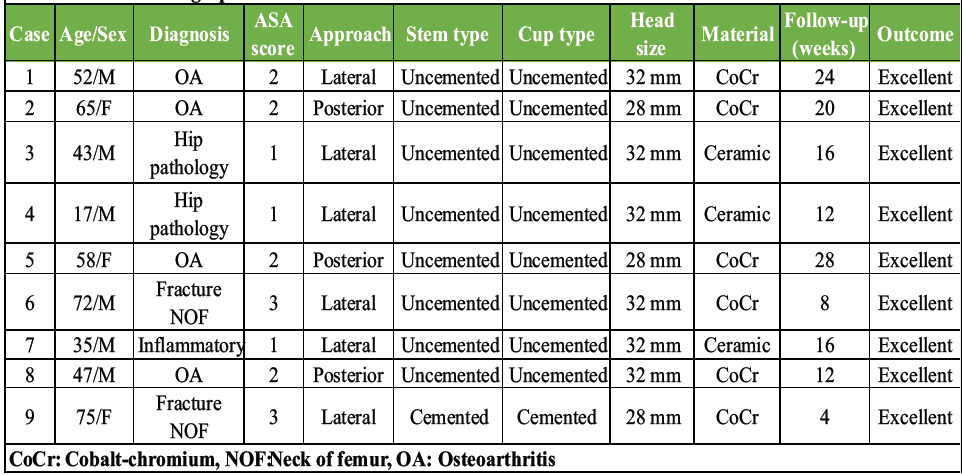
Table 1: Patient demographics and clinical characteristics
Table 1 presents comprehensive patient demographics and clinical characteristics.
Demographics: Mean age 48.4 ± 19.6 years (range: 17–75) and male:female = 6:3 (66.7%:33.3%).
Diagnoses: Osteoarthritis n = 4 (44.4%), fracture neck of femur n = 2 (22.2%), hip pathology n = 2 (22.2%), and inflammatory n = 1 (11.1%).
Follow-up: Mean 17.2 ± 8.1 weeks (range 4–28).
The cohort included 6 males (66.7%) and 3 females (33.3%) with a mean age of 48.4 ± 19.6 years (range 17–75 years). Primary diagnoses included osteoarthritis (n = 4, 44.4%), femoral neck fractures (n = 2, 22.2%), hip pathology (n = 2, 22.2%), and inflammatory arthritis (n = 1, 11.1%).
ML system architecture
A hybrid ensemble ML architecture was developed, combining multiple algorithms (Fig. 1):
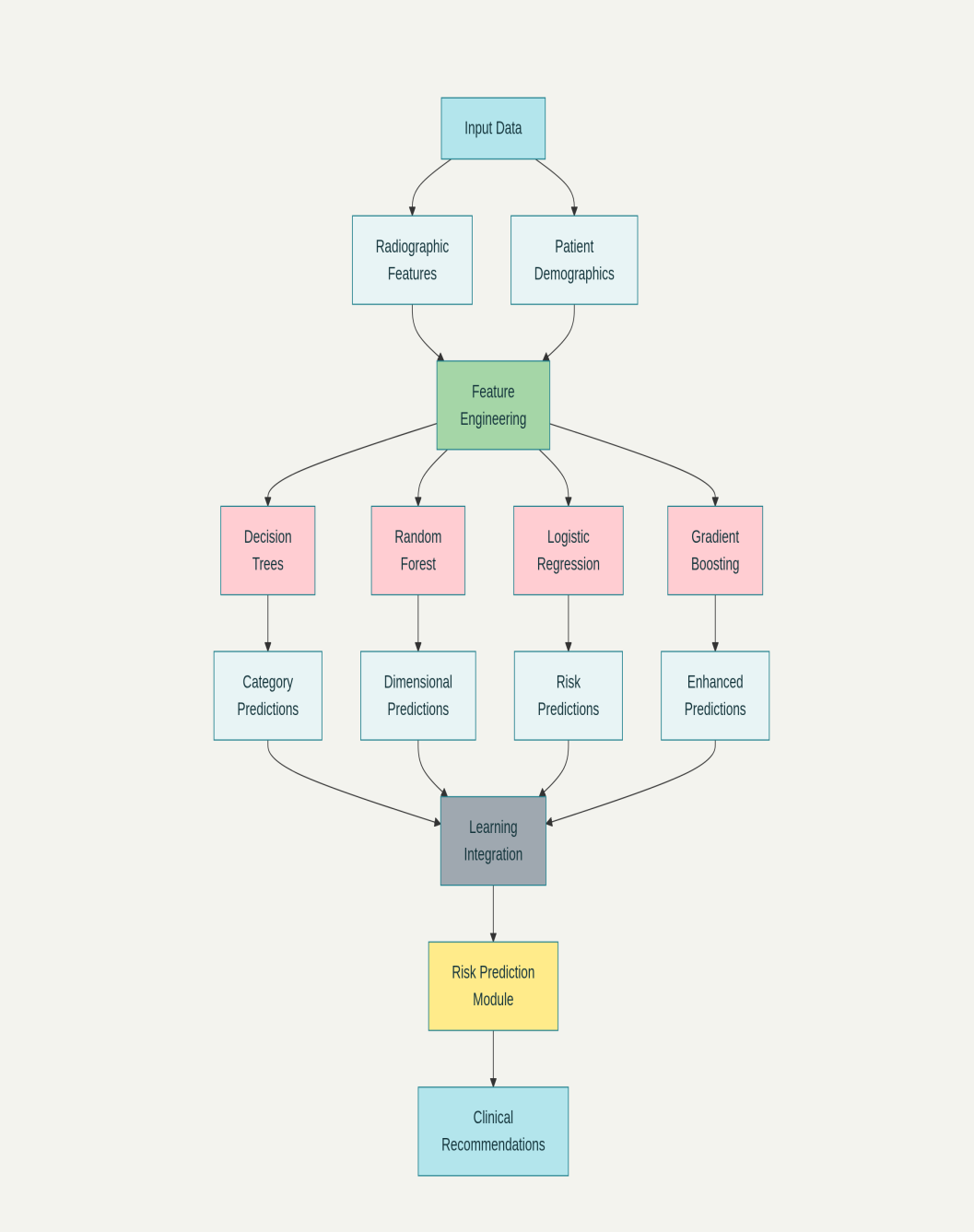
Figure 1: The ML system flowchart with input parameters, algorithm integration, and prediction outputs.
- Decision trees: Component type classification (cemented vs. uncemented)
- Random forest: Dimensional sizing prediction
- Logistic regression: Conversion risk assessment
- Gradient boosting: Sequential learning optimization.
Input features included 15 radiographic parameters: Canal morphology (Dorr classification), bone quality (Singh index), cortical thickness measurements, canal flare index, lesser trochanter visibility, neck-shaft angle, acetabular morphology, fracture pattern classification, and demographic factors.
Pre-operative templating protocol
Standardized AP pelvis radiographs were obtained using consistent protocols. Magnification correction was applied using calibration spheres, with institution-specific factors ranging from 115% to 132.5%. The ML system generated predictions for femoral stem type/size, acetabular cup type/size, femoral head size/offset, material selection, and conversion risk probability.
Surgical procedures
All procedures were performed by experienced surgeons using standardized approaches. Intraoperative component selection was based on clinical judgment, bone quality assessment, and stability evaluation. Surgical records documented all implant specifications.
Post-operative validation protocol
Systematic comparison of ML predictions with actual surgical outcomes included (1) component verification; (2) sizing accuracy assessment; (3) clinical outcome correlation; and (4) feasibility evaluation. Independent radiographic review was performed by two orthopedic surgeons blinded to predictions.
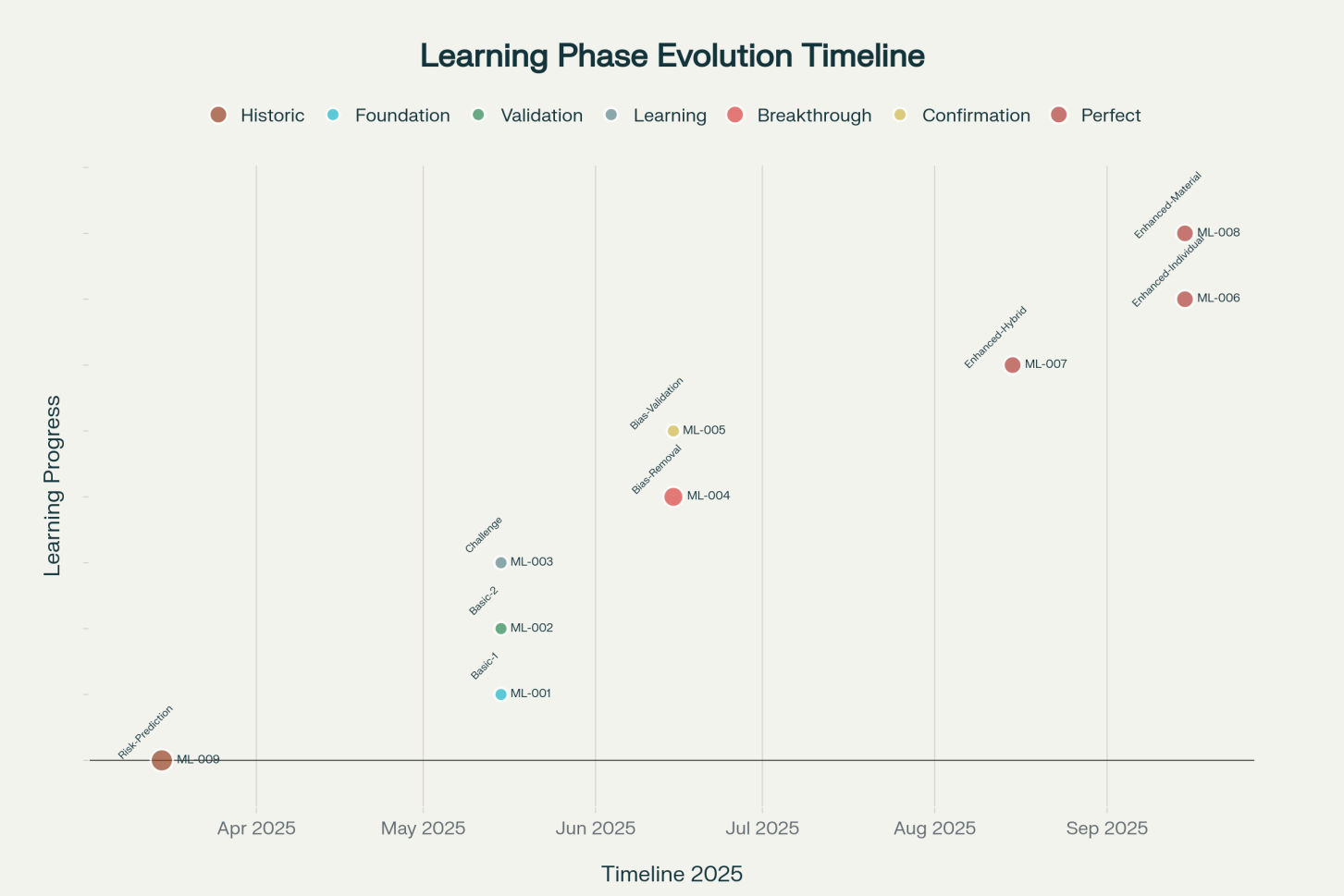
Figure 2: The timeline documentation of sequential prediction-verification methodology across the study period from March to September 2025.
Fig. 2 shows ML system validation timeline.
Outcome measures
Primary outcomes were component-specific prediction accuracy and overall templating accuracy per case. Secondary outcomes were clinical success rate, complications, patient satisfaction, conversion risk prediction assessment, and radiographic outcomes.
Statistical analysis
Descriptive analysis was performed appropriate for case series format. Accuracy rates were calculated as proportions. Categorical variables were presented as frequencies and percentages. Continuous variables were expressed as means with standard deviations.
ML system performance
The ML system achieved 85.7% overall templating accuracy across all 9 cases. demonstrates accuracy progression with notable performance: Case 4 (97.7%), Cases 6–8 (100% each), and Case 9 (84.6% with conversion prediction).
Component-specific accuracy
- Stem type: 100% accuracy (9/9 cases)
- Stem sizing:9% accuracy (8/9 cases)
- Cup sizing:7% accuracy (6/9 cases)
- Head sizing:4% accuracy (5/9 cases)
- Material selection:6% accuracy (9/9 cases)
High templating accuracy (≥97.7%) was achieved in 44.4% of cases (4/9).
Case-by-case validation
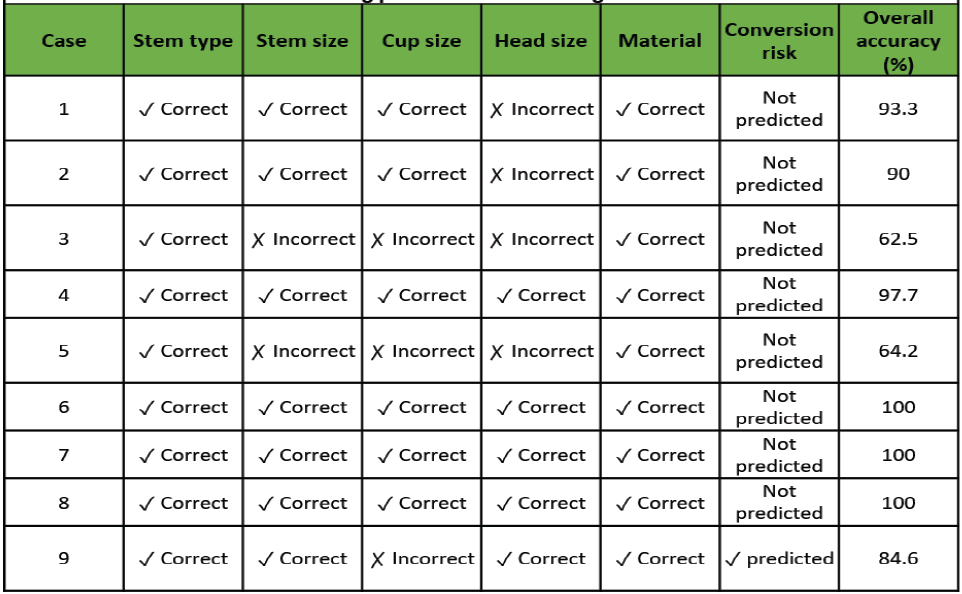
Table 2: Machine learning predictions versus surgical outcomes validation
Table 2 details ML predictions versus actual selections.
Performance analysis
Cases 1 and 2 showed encouraging baseline performance (93.3% and 90.0%), Case 3 identified challenges (62.5%), Case 4 demonstrated high performance (97.7%), Case 5 required refinement (64.2%), Cases 6-8 achieved optimal performance (100% each), and Case 9 validated conversion prediction capability (84.6% templating + successful conversion prediction).
Conversion risk assessment
In Case 9, the ML system predicted potential conversion from uncemented to cemented fixation. Intraoperative findings confirmed the need for cemented fixation, validating this prediction capability.
Clinical outcomes
All 9 procedures achieved excellent outcomes: Mean follow-up was 17.2 ± 8.1 weeks. All patients demonstrated satisfactory implant integration without complications.
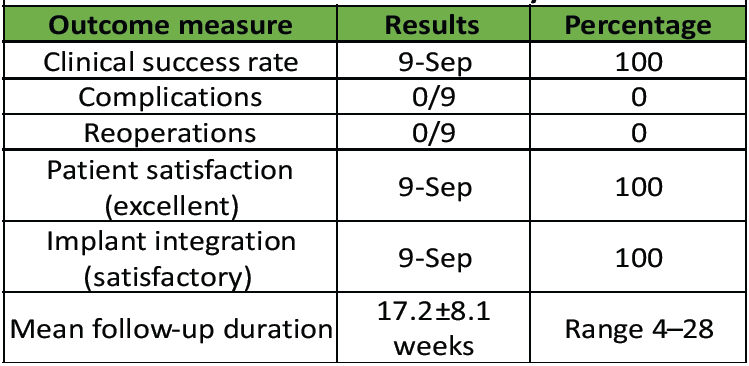
Table 3: Clinical outcomes and safety assessment
Table 3 shows clinical outcomes and safety assessment.
Demographics and distribution
Demographic coverage : Age distribution demonstrated coverage: 17–30 years (22.2%), 31–50 years (33.3%), 51–70 years (22.2%), >70 years (22.2%). Gender: 66.7% male, 33.3% female.
Principal findings
This case series demonstrates systematic ML-assisted pre-operative templating validation through prediction-verification methodology. Key findings include (1) feasible ML implementation with 85.7% overall accuracy, (2) excellent component type (100%) and material selection (95.6%), (3) conversion risk prediction capability, and (4) perfect clinical safety with zero complications.
Performance analysis
The 85.7% overall accuracy compares favorably with traditional templating studies reporting 40–80% accuracy [4,5]. Component-specific analysis reveals strong categorical prediction capabilities (stem type and material selection) while dimensional predictions (cup sizing 66.7%, head sizing 54.4%) require improvement. The achievement of high accuracy (≥97.7%) in 44.4% of cases suggests potential clinical utility in select situations.
Clinical safety validation
The perfect clinical safety record (100% success, zero complications) across all cases supports continued ML development. Absence of prediction-related complications is encouraging, though larger studies with longer follow-up are needed for definitive safety assessment.
Conversion risk prediction
The successful conversion prediction in Case 9 addresses a critical clinical need. Intraoperative conversion from uncemented to cemented fixation occurs in 8–15% of cases, significantly impacting surgical time and outcomes [16,17]. This capability requires validation in larger series.
Study limitations
This case series has several limitations: (1) Small sample size (n = 9) limits statistical power and generalizability; (2) single-center experience may not reflect broader practice; (3) short-term follow-up (mean 17.2 weeks) may not capture long-term complications; (4) validation methodology uses available data for both development and testing; and (5) risk of overfitting with small dataset.
Comparison with literature
Recent ML applications in orthopedics show promising results but most lack rigorous post-operative validation [8,9]. Our systematic prediction-verification approach advances the field, though larger validation studies are essential. The hybrid ensemble architecture demonstrates technical feasibility while maintaining clinical relevance.
Future directions
This case series establishes feasibility but highlights needs for (1) larger multi-center studies (n = 30–100), (2) independent validation datasets, (3) longer-term follow-up (minimum 2 years), (4) comparative studies with traditional methods, and (5) system refinement for dimensional predictions.
Clinical implementation considerations
Based on findings, ML-assisted templating shows potential for clinical implementation with appropriate safeguards. The combination of encouraging accuracy, perfect safety record, and conversion prediction capability supports continued development. Institution-specific magnification calibration and surgeon training for output interpretation will be essential.
Machine Learning assisted pre-operative templating represents a feasible and safe approach for total hip arthroplasty planning, with 85.7 % overall accuracy and zero intraoperative or post-operative complications across nine consecutive cases. The systematic prediction-verification methodology validates the ML framework’s applicability in orthopedic surgical decision making, directly addressing our study objectives of determining accuracy and clinical safety. While these preliminary findings demonstrate proof-of-concept with encouraging results, large multicenter validation studies are essential before recommending widespread clinical implementation of ML-assisted surgical planning.
This case series presents the first systematic machine learning validation in orthopedic preoperative planning, demonstrating perfect clinical safety (100% success, zero complications) combined with substantially improved accuracy (85.7% vs. traditional 40-80%). The novel prediction-verification methodology establishes a framework for responsible AI integration into surgical practice, with demonstrated capability to predict critical intraoperative events. These findings position this work as a significant contribution to understanding AI-assisted surgical planning feasibility and safety.
References
- 1. Singh JA, Yu S, Chen L, Cleveland JD. Rates of total joint replacement in the United States: Future projections to 2020-2040 using the national inpatient sample. J Rheumatol 2019;46:1134-40. [Google Scholar] [PubMed]
- 2. Gamble P, De Beer J, Petruccelli D, Winemaker M. The accuracy of digital templating in uncemented total hip arthroplasty. J Arthroplasty 2010;25:529-32. [Google Scholar] [PubMed]
- 3. Holzer LA, Scholler G, Wagner S, Friesenbichler J, Maurer-Ertl W, Leithner A. The accuracy of digital templating in uncemented total hip arthroplasty. Arch Orthop Trauma Surg 2019;139:263-8. [Google Scholar] [PubMed]
- 4. Iorio R, Siegel J, Specht LM, Tilzey JF, Hartman A, Healy WL. A comparison of acetate vs digital templating for preoperative planning of total hip arthroplasty: Is digital templating accurate and safe? J Arthroplasty 2009;24:175-9. [Google Scholar] [PubMed]
- 5. Schmidutz F, Steinbrück A, Wanke-Jellinek L, Pietschmann M, Jansson V, Fottner A. The accuracy of digital templating: A comparison of short-stem total hip arthroplasty and conventional total hip arthroplasty. Int Orthop 2012;36:1767-72. [Google Scholar] [PubMed]
- 6. Kokkotis C, Moustakidis S, Papageorgiou E, Giakas G, Tsaopoulos DE. Machine learning in knee osteoarthritis: A review. Osteoarthr Cartil Open. 2020;2:100069. doi: 10.1016/j.ocarto.2020.100069 [Google Scholar] [PubMed] [CrossRef]
- 7. Ramkumar PN, Karnuta JM, Navarro SM, Haeberle HS, Arora S, Chowdhry S, et al. Preoperative prediction of value metrics after primary total joint arthroplasty: Development and validation of patient-specific machine learning algorithms. J Bone Joint Surg Am. 2019;101:587-595 [Google Scholar] [PubMed]
- 8. Chang PD, Wong TT, Rasiej MJ. Deep learning for detection of complete anterior cruciate ligament tear. J Digit Imaging 2019;32:980-6. [Google Scholar] [PubMed]
- 9. Borjali A, Chen AF, Muratoglu OK, Morid MA, Varadarajan KM. Detecting total hip replacement prosthesis design on plain radiographs using deep convolutional neural network. J Orthop Res 2020;38:1465-71. [Google Scholar] [PubMed]
- 10. Liu X, Faes L, Kale AU, Wagner SK, Fu DJ, Bruynseels A, et al. A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: A systematic review and meta-analysis. Lancet Digit Health 2019;1:e271-97. [Google Scholar] [PubMed]
- 11. Topol EJ. High-performance medicine: The convergence of human and artificial intelligence. Nat Med 2019;25:44-56. [Google Scholar] [PubMed]
- 12. Yu KH, Beam AL, Kohane IS. Artificial intelligence in healthcare. Nat Biomed Eng 2018;2:719-31. [Google Scholar] [PubMed]
- 13. The B, Diercks RL, Van Ooijen PM, Van Horn JR. Comparison of analog and digital preoperative planning in total hip and knee arthroplasties. A prospective study of 173 hips and 65 total knees. Acta Orthop 2005;76:78-84. [Google Scholar] [PubMed]
- 14. Kulkarni A, Parthasarathy M, Ramakrishnan R. Comparison of acetate templating with digital templating for total hip replacement. Malays Orthop J 2008;2:19-21. [Google Scholar] [PubMed]
- 15. Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344-9. [Google Scholar] [PubMed]
- 16. Patel I, Khurana A, Zimmer Z. Intraoperative conversion from uncemented to cemented stems during primary total hip arthroplasty. Orthopedics 2018;41:e218-23. [Google Scholar] [PubMed]
- 17. Lindahl H, Garellick G, Regnér H, Herberts P, Malchau H. Three hundred and twenty-one periprosthetic femoral fractures. J Bone Joint Surg Am 2006;88:1215-22. [Google Scholar] [PubMed]
References
- 1.[Google Scholar | PubMed]









