[box type=”bio”] Learning Point of the Article: [/box]
Denosumab therapy for spinal giant-cell tumor of bone (GCTB)-induced tumor reduction and realignment ofthe vertebral column; however, a long follow-up period is imperative because denosumab does not completely eliminate the GCTB cells.
Case Report | Volume 10 | Issue 2 | JOCR March – April 2020 | Page 76-79 | Takafumi Yayama, Kanji Mori, Akira Nakamura, Tomohiro Mimura, Shinji Imai. DOI: 10.13107/jocr.2020.v10.i02.1706
Authors: Takafumi Yayama[1], Kanji Mori[1], Akira Nakamura[1], Tomohiro Mimura[1], Shinji Imai[1]
[1]Department of Orthopaedic Surgery, Shiga University of Medical Science, Seta Tsukinowa, Otsu 520-2192, Shiga, Japan.
Address of Correspondence:
Dr. Takafumi Yayama,
Department of Orthopaedic Surgery, Shiga University of Medical Science, Seta Tsukinowa, Otsu, Shiga 520-2192, Japan.
E-mail: yayama@belle.shiga-med.ac.jp
Abstract
Introduction: Denosumab, a monoclonal antibody that inhibits the receptor activator of nuclear factor-kappa? (RANK) ligand, has been reported to reduce tumor size and progression, promote bone mineralization reconstruction, and increase bone density in patients with giant-cell tumor of bone (GCTB). However, information regarding the histopathological findings of spinal GCTB following denosumab therapy and the time course of the treatment is limited.
Case Report: We report the case of a 58-year-old woman with progressive low back pain for 3 months before admission. Radiological and histological examinations revealed L4 GCTB. The patients received 10 courses of denosumab, and the tumor was subsequently resected. The therapy resulted in reduction of tumor mass and replacement of the lesions with bone tissue, particularly at the extravertebral and intracanal mass lesions. Histological examination of resected vertebra revealed a notable decrease in the number of RANK-positive and cyclooxygenase-2-positive cells. However, few RANK-positive cells were present around the woven bone.
Conclusion: Denosumab therapy for spinal GCTB is effective for reducing the tumor stage, surgical complications, and neurological impairment progression; however, it does not lead to total elimination of GCT cells, and careful consideration is needed in terms of the surgical procedure and post-operative denosumab therapy.
Keywords: Denosumab, giant-cell tumor, immunohistochemistry.
Introduction
Giant-cell tumor of bone (GCTB) is defined as an intermediate and locally aggressive tumor, which exhibits osteoclastic activity and commonly occurs at the metaphyseal and epiphyseal regions of long bones. Histologically, GCTBs are composed of mononuclear stromal cells and multinucleated giant cells and have a propensity for local recurrence and metastasis [1, 2]. The treatment of GCTB is highly controversial. The primary treatment method involves complete surgical resection; however, effective treatment options are limited for patients with lesions in locations that are not amenable to surgical resection [3]. In particular, spinal GCTB is associated with a high risk of morbidity and neurological impairment due to the proximity of the tumor to the spinal cord and nerve roots [4].
Denosumab, a receptor activator of nuclear factor-kappa ligand (RANKL) inhibitor, is a fully human monoclonal antibody that binds to RANKL and directly inhibits osteoclastogenesis. Denosumab has been approved for the treatment of GCTB in adults and skeletally mature adolescents [5]. Denosumab causes objective changes in clinical response in patients with non-resectable or highly recurrent tumors [6]. In addition, it prevents tumor progression, reduces tumor size, reconstructs mineralized bone, and increases intralesional bone density [5, 7]. However, there is limited information on the clinical course of denosumab therapy for spinal GCTB or on the histopathological evaluation of the effectiveness of the therapy is required. In this study, we describe the case of a 58-year-old woman with L4 GCTB. We performed an immunohistochemical examination of the resected spinal tissue and provided evidence supporting the use of denosumab therapy for spinal GCTB.
Case Report
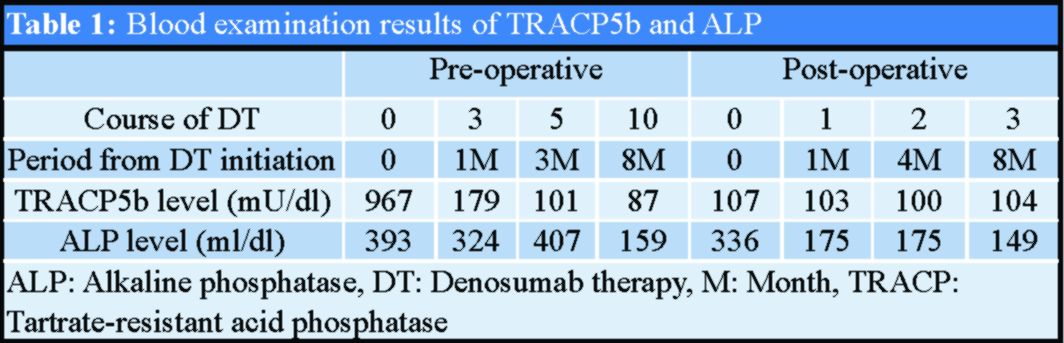
The patient was a 58-year-old woman who presented with progressive low back pain for 3 months before admission. She had no history of hypertension, diabetes mellitus, systemic carcinoma, or congenital tissue abnormalities. On admission, she exhibited marked gait disturbance with low back pain and sciatic nerve pain, which was dominant on her right side. The strength of the right tibialis anterior and extensor hallucis longus muscles was Grade 3–4, and sensory disturbance, including pain as well as thermal and touch sensations, occurred on the bilateral lower thigh. Findings of deep tendon reflexes and pathological reflexes were normal. Blood examination results revealed a tartrate-resistant acid phosphatase (TRACP)5b level of 967 mU/dl and an alkaline phosphatase (ALP) level of 393 ml/dl (Table 1). Initial X-ray and computed tomography (CT) results revealed the presence of an osteolytic lesion involving the L4 vertebra with the abnormal alignment of anterior spondylolisthesis (Fig. 1a and b). Magnetic resonance imaging (MRI) revealed low signal intensity and intermediate-to-high signal intensity areas on T1- and T2-weighted images, respectively.
Gadolinium-enhanced axial images revealed that the tumor mass expanded into the extravertebral and intraspinal canal spaces (Fig. 1c-e). Results of CT-guided percutaneous biopsy were consistent with the diagnosis of giant-cell tumor of the spine. As per the protocol in a previous study, the patient was preoperatively administered 10 courses of denosumab (120 mg at a time) in monthly cycles with initial additional loading doses [8, 9]. Following denosumab therapy, her severe low back pain was resolved and no neurological deficits were noted. Follow-up pre-operative X-ray and CT revealed progression of anterior deviation and an increase in vertebral body mineralization (Fig. 1f and g). MRI revealed a decrease in tumor enhancement and a marked reduction in the tumor size in the retroperitoneum space and intraspinal canal region (Fig. 1h-j). The TRACP5b and ALP levels returned to normal after 3and 10courses of denosumab therapy, respectively (Table 1).
A combined anterior and posterior approach was used to perform the total spondylectomy of the L4 vertebra. First, we exposed the dura mater by en bloclaminectomy and detached the bilateral L4 pedicles using a threaded T-saw. The lamina and pedicles were rigid, and there was no adhesion between the dura mater and tumor section. The lateral aspect of the L4 vertebral body was exposed, and disc resection was performed using the posterior incision approach. Using the anterolateral approach, the vertebral body was resected piece by piece, and the bone in the regenerated cortical GCTB was treated with denosumab. Furthermore, corpectomy of L4 was performed; subsequently, titanium cage reconstruction was performed with posterior L3-L5 stabilization. Postoperatively, the patient remains pain free without neurological symptoms, and there is no radiologic evidence of tumor recurrence for 1 year after surgery, i.e., following three courses of denosumab administered at 2-month intervals. Light microscopy revealed numerous multinucleated giant cells in the background of neoplastic mononuclear stromal cells in pre-operative biopsy samples (Fig. 2a). Immunohistochemical staining revealed RANK-positive mononuclear cells and aggregations of strongly cyclooxygenase-2 (COX-2)-positive mononuclear stromal cells (Fig. 2b and c). The microscopic examination of tissues harvested after denosumab therapy revealed a fibrous matrix with no multinucleated giant cells (Fig. 2d). Immunohistochemistry detected RANK-positive cells around the woven bone, although almost all of the RANK and COX-2-positive cells disappeared (Fig. 2e and f).
Discussion
The primary curative treatments for GCTB comprise complete surgical resection and adequate local control. Local recurrence develops after several years in approximately 10%–50% of patients and after intralesional treatment or wide resection in 5% of patients [3, 10]. Effective intervention and treatment options are limited for patients with lesions in locations that are not amenable to surgical resection, such as the spinal area. A recent report revealed the effectiveness of denosumab therapy for spinal GCTB; they reported 10% reduction in tumor size and a nearly complete radiological response [11]. Further, denosumab therapy led to shrinkage of epidural extraosseous GCTB as well as the progression of vertebral collapse and the formation of massive calli [8] in the spinal vertebral body of patients with GCTB. In the present study, denosumab therapy markedly reduced the extravertebral and intracanal tumor mass lesions and improved pre-operative neurological impairment. The vertebral body showed sclerotic changes, indicating bone remodeling. The use of denosumab therapy for GCTB is experimental and is based on the pathology of the disease. A systematic review of 686 patients with GCTB after denosumab therapy, including 55% of primary and 45% of recurrent cases, revealed a recurrence rate of 2% [9]. Another study that evaluated 40 patients treated with curettage and perioperative denosumab reported recurrence rates of 6/21 (28.6%), 2/9 (22.2%), and 0/10 (0.0%) in the pre-operative, post-operative, and both pre- and post-operative denosumab therapy groups, respectively; however, 34 of 158 patients (21.5%) treated with only curettage had local and distant recurrence [12]. It is evident that denosumab therapy is useful in neoadjuvant settings; however, its optimal treatment duration remains unknown. Our case presented no radiologic evidence of tumor recurrence for 1 year after total tumor resection with 10 pre-operative courses of denosumab; however, a longer follow-up is imperative, especially for inaccessible areas. Radiological findings revealed that the median time to objective tumor response was 3 months [13]. The previous studies have reported that the pre-operative use of denosumab over 6 months can improve the treatment outcomes of GCTB and its recurrence [9] and that more than five courses of pre-operative denosumab therapy are significantly associated with decreased incidence of local recurrence after curettage [12]. In this case, post-operative denosumab therapy was performed because the vertebral body was resected piece by piece during the L4 corpectomy; however, its adaptation or protocol has not been established. The duration of denosumab was reduced every other month due to the patient’s physical burdens, costs, and no indications of a tumor lesion in the post-operative radiographs. GCTBs are characterized by osteolytic phenotypes. RANK/RANKL signaling induces interaction between RANKL-expressing mesenchymal spindle-like stromal cells and osteoclast giant cells, resulting in bone resorption. Denosumab binds to RANKL, thereby inhibiting RANK/RANKL signaling and directly inhibiting osteoclast activation [2, 5]. It is important to note that our pathological findings revealed the presence of RANK-positive cells around the woven bone despite a significant reduction in the number of these cells after denosumab therapy. Similar findings were also reported following adequate denosumab therapy [8]. These results suggest the necessity of post-operative denosumab therapy because the pre-operative approach leads to incomplete inhibition of RANK/RANKL signaling in GCTB. COX-2 is a chemical mediator involved in the metabolism of arachidonic acid, which is mainly expressed in endothelial cells or vascular smooth muscle cells in response to tissue inflammation, with limited expression in normal tissues. During bone metabolism, COX-2 is highly activated in response to both tissue inflammation and inhibition of the osteogenetic process [14], and this activation is accompanied by an increase in the expression of RANKL in osteoblasts or synovial cells through the production of prostaglandins. Recent reports [15] have demonstrated the coactivation of tumor necrosis factor (TNF)-alpha, RANKL, and COX-2. The upregulation of TNF-alpha signaling increases the expression of RANKL and COX-2. This case demonstrated the improvement of low back pain in a patient with GCTB following tumor reduction. These improvements may be induced by realignment of the biomechanical alignment of the vertebral column and a halt in the osteoclastic activity associated with a decreased expression of chemical factors.
Conclusion
Denosumab therapy reduced the tumor mass and induced bone formation in patients with spinal GCTB. In particular, the extravertebral and intracanal tumor mass lesions were markedly reduced. In addition, denosumab reduced the tumor stage, surgical complications, and the progression of neurological impairment. In this study, the histopathological findings of the resected sections after 10 courses of denosumab therapy revealed the presence of RANK-positive cells around the woven bone. These results suggest that denosumab does not completely eliminate GCTB cells; thus, surgeons must consider effective operative procedures and the use of post-operative denosumab therapy.
Clinical Message
Denosumab therapy can improve the treatment of spinal GCTB. The tumor reduction and realignment of the vertebral column were acquired; however, a long post-operative follow-up period is imperative because denosumab does not completely eliminate the presence of GCTB cells.
References
1. EnnekingWF. A system of staging musculoskeletal neoplasms.Clin OrthopRelat Res1986;204:9-24.
2. Harrop JS, Schmidt MH, Boriani S, Shaffrey CI. Aggressive benign primary spine neoplasms: Osteoblastoma, aneurysmal bone cyst, and giant cell tumor. Spine (Phila Pa 1976) 2009;34 Suppl 22:S39-47.
3. KlenkeFM, WengerDE, InwardsCY, RosePS, SimFH. Recurrent giant cell tumor of long bones: Analysis of surgical management.Clin OrthopRelat Res2011;469:1181-7.
4. ThomasDM, SkubitzKM. Giant cell tumour of bone.CurrOpin Oncol2009;21:338-44.
5. Chawla S, Henshaw R, Seeger L, Choy E, Blay JY, Ferrari S, et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: Interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol 2013;14:901-8.
6. Xu SF, Adams B, Yu XC, Xu M. Denosumab and giant cell tumour of bone: A review and future management considerations. Curr Oncol 2013;20:E442-7.
7. BranstetterDG, NelsonSD, ManivelJC, BlayJY, ChawlaS, ThomasDM, et al. Denosumab induces tumor reduction and bone formation in patients with giant-cell tumor of bone.Clin Cancer Res2012;18:4415-24.
8. YonezawaN, MurakamiH, KatoS, TakeuchiA, TsuchiyaH. Giant cell tumor of the thoracic spine completely removed by total spondylectomy after neoadjuvant denosumab therapy.Eur Spine J2017;26:236-42.
9. JamshidiK, GharehdaghiM, HajialilooSS, MirkazemiM, GhaffarzadehganK, IzanlooA. Denosumab in patients with giant cell tumor and its recurrence: A systematic review.Arch Bone Jt Surg2018;6:260-8.
10. ArbeitsgemeinschaftKnochentumoren, BeckerWT, DohleJ, BerndL, BraunA, CserhatiM, et al. Local recurrence of giant cell tumor of bone after intralesional treatment with and without adjuvant therapy.J Bone Joint Surg Am2008;90:1060-7.
11. GoldschlagerT, DeaN, BoydM, ReynoldsJ, PatelS, RhinesLD, et al. Giant cell tumors of the spine: Has denosumab changed the treatment paradigm?J Neurosurg Spine2015;22:526-33.
12. UrakawaH, YonemotoT, MatsumotoS, TakagiT, AsanumaK, WatanukiM, et al. Clinical outcome of primary giant cell tumor of bone after curettage with or without perioperative denosumab in Japan: From a questionnaire for JCOG 1610 study.World J Surg Oncol2018;16:160.
13. EngellauJ, SeegerL, GrimerR, HenshawR, GelderblomH, ChoyE, et al. Assessment of denosumab treatment effects and imaging response in patients with giant cell tumor of bone.World J Surg Oncol2018;16:191.
14. JanssenMP, CaronMM, vanRietbergen B, SurtelDA, vanRhijn LW, WeltingTJ, et al. Impairment of the chondrogenic phase of endochondral ossification in vivo by inhibition of cyclooxygenase-2.Eur Cell Mater2017;34:202-16.
15. Park HJ, Baek K, Baek JH, Kim HR. TNF α increases RANKL expression via PGE2-induced activation of NFATc1. Int J Mol Sci 2017;18:E495.
 |
| Dr. Takafumi Yayama |
| How to Cite This Article: Yayama T, Mori K, Nakamura A, Mimura T, Imai S. Denosumab Therapy for Giant-cell Tumor of the Lumbar Spine: A Case Report and Immunohistochemical Examination. Journal of Orthopaedic Case Reports 2020 Mar-Apr;10(2): 76-79. |
[Full Text HTML] [Full Text PDF] [XML]
[rate_this_page]
Dear Reader, We are very excited about New Features in JOCR. Please do let us know what you think by Clicking on the Sliding “Feedback Form” button on the <<< left of the page or sending a mail to us at editor.jocr@gmail.com