Although S. paucimobilis is an uncommon nosocomial infection, it should be kept in mind as a cause, considering its ubiquitous presence in the environment, especially in immunocompromised patients and those with co-morbidities.
Dr. Nandan Marathe,
Department of Spine Services,
Indian Spinal Injuries Centre, Vasant Kunj, Sector C, New Delhi -110 070, India.
E-mail: nandanmarathe88@gmail.com
Introduction: Sphingomonas paucimobilis is an opportunistic pathogen and a rare cause of human infection. This case report shows bacteremia with pyogenic spondylodiscitis in lumbosacral spine caused by Sphingomonas and discusses its clinical diagnosis, treatment, and literature reviews.
Case Report: Patient presented with severe low back pain, inability to walk and fever following a L5-S1 decompression elsewhere, which corresponded clinico-radiologically with a picture of a spondylodiscitis on radiographs, magnetic resonance imaging, and computed tomography. His blood culture was positive for S. paucimobilis. He was treated successfully by surgical debridement, stabilization, and fusion along with intravenous Linezolid followed by oral therapy, based on the antibiotic susceptibility profile. Repeat blood culture was negative after 7 weeks of antibiotic therapy. Patient improved symptomatically with radiographs showing good fusion at 1 year follow-up. S. paucimobilis, though a low virulence organism, is an emerging pathogen and should be dealt with cautiously.
Conclusion: This reiterates the importance of culture as unusual organisms may be isolated and appropriate antibiotics form the mainstay of treatment.
Keywords: Sphingomonas paucimobilis, spondylodiscitis, spinal fusion, linezolid.
Sphingomonas paucimobilis is an aerobic, non-fermenting, non-spore-forming, oxidase positive, Gram-negative bacillus. It has a characteristic feature of forming yellow pigment colonies. It is an opportunistic pathogen and an uncommon source of infection in humans [1, 2]. It usually infects immunocompromised individuals. Non-fermenting Gram-negative organisms are a major source of opportunistic nosocomial infections. The major bacteria in this group are: Acinetobacter baumannii, Burkholderia cepacia, Pseudomonas aeruginosa, Ralstonia pickettii, and Stenotrophomonas maltophilia. S. paucimobilis an emerging pathogen which has clinically been overlooked as an infecting organism due to its low virulence [3]. Several case reports and case series of S. paucimobilis infection have been published including meningitis, septicemia, empyema, cellulitis, urinary tract infections, cervical adenitis, peritonitis and abscesses in brain, and spleen and pancreas [4, 5, 6, 7, 8, 9, 10, 11, 12]. Only three cases of osteomyelitis are recorded in the literature, of which two were in immunocompromised patients [13, 14, 15]. Recently one case of pyogenic spondylodiscitis (PS) in lumbar spine was reported which was managed conservatively with antibiotics alone for 7 weeks [16]. We report lumbosacral PS caused by S. paucimobilis, which was managed by spinal fusion. To the best of our knowledge, this is the 1st instance of S. paucimobilis managed by surgical intervention.
A 45-year-old male patient of East African origin presented to our tertiary care spine institute with complains of low back pain. Patient had difficulty in walking or standing and he could barely sit erect for 15 min. Visual analog scale (VAS) score for back pain was 8. Pain was only relieved on lying down. There were no radicular symptoms. He had a previous history of disk prolapse at L5-S1 level and was operated elsewhere for the same with an open discectomy. However, post-procedure, his pain had increased to such an extent that he was unable to mobilize without support. He had severe febrile illness for 1 week followed by a low grade fever that was persistent at the time of presentation to our institute. The patient was a known case of diabetes mellitus for 3 years, medicated with a single, oral glucose-lowering agent. Patient presented to us 1 month post the first surgery.
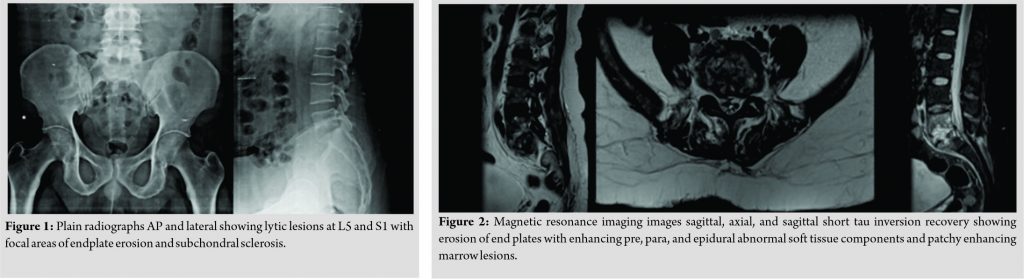
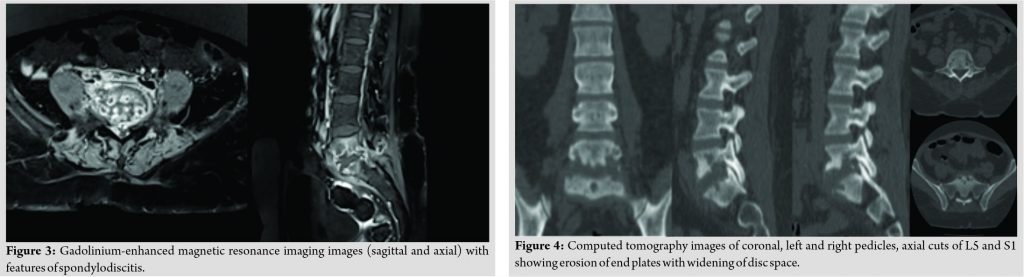
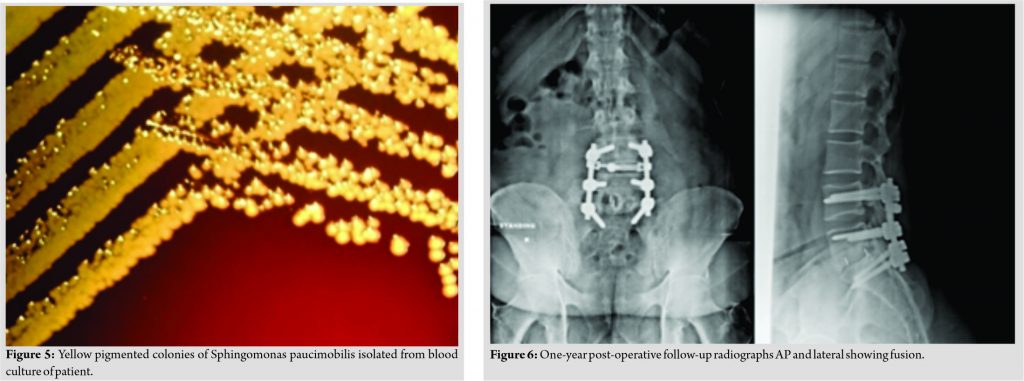
The patient had midline and para-spinal tenderness over L5-S1 region. Movements of the spine were grossly restricted due to pain. The patient was afebrile. On admission, the blood investigations showed an erythrocyte sedimentation rate of 65, C-reactive protein of 34, and total leukocyte count 10700. Radiographs of the lumbosacral spine revealed lytic lesions at L5 and S1 with focal areas of endplate erosion and subchondral sclerosis which was compatible with diagnosis of PS at L5-S1 (Fig. 1). Magnetic resonance imaging (MRI) showed erosion of end plates with enhancing pre, para, and epidural abnormal soft tissue components and patchy enhancing marrow lesions at L5 and S1 (Fig. 2). The diagnosis was further confirmed on the gadolinium contrast-enhanced MRI (Fig. 3). Computed tomography (Fig. 4) findings of the lumbosacral spine corroborated with the plane radiograph and MRI. Blood and urine cultures were sent to look for any other source of infection. Blood culture in our case grew S. paucimobilis (Fig. 5). The recovery of S. paucimobilis from specimens was accomplished by the processing of blood cultures in a Bactec Model 9240 (Becton-Dickinson) or BacT/ALERT 3D. Identification of S. paucimobilis and antibiotic sensitivity tests was done on the Vitek II automated system.
The patient was treated by posterior decompression and stabilization from L4 to S1 and inter body and posterolateral inter transverse bone grafting at L5-S1. Inter body fusion was accomplished by a tricortical bone graft from iliac crest on one side and polyether ether ketone (PEEK) cage filled with local bone graft from the other due to the large bony defect. Intra-operatively, disk material and bony tissue from L5-S1 disk space and vertebral bodies were sent for histopathology and culture sensitivity studies. Post-procedure course was uneventful, the patient improved symptomatically and was mobilized on 2nd post-operative day with a walker. Patient was simultaneously started on Injection Linezolid 600 mg intravenous (IV) twice a day as per the antibiotic sensitivity of the blood culture report. Cultures from the disk tissue were negative for any organism. Patient was discharged on 6th post-operative day and IV antibiotics were continued for a month, followed by oral antibiotics for 3 weeks (Tab. Linezolid 600 mg twice daily). His VAS score for back pain improved to 2. A blood culture repeated after 7 weeks did not show growth of any organism. Patient was comfortable and performing all his routine activities without support. Follow-up radiographs showed good radiological fusion without any features suggestive of further infective process (Fig. 6).
S. paucimobilis, initially called Pseudomonas paucimobilis, was renamed on basis of phylogenetic data. It was thus named because it has of its slow motility due to one polar flagella [1, 2, 3, 14, 15, 16, 17, 18, 19, 20, 21, 22]. S. paucimobilis has been isolated from different sources, including water system, respiratory-therapy equipment, indwelling devices, and various clinical specimens in hospital. It is found to form a biofilm in water pipe systems [14, 20, 21]. Bacteremia caused by this group of Gram-negative organisms can be fatal. However, it is not so with S. paucimobilis probably due to the lack of typical lipopolysaccharide constituent of cellular membrane associated with endotoxin activity. Most of the S. paucimobilis infections published in literature have been health-care associated infections. This bacterium causes two types of infection: Community acquired and nosocomial. Community acquired infections are usually of environmental origin. Nosocomial infection is commonly due to contaminated hospital fluids [23]. Community acquired infection can be defined as bacteremia that occurs within 72-h of hospitalization [24]. As per Cheong et al. [25], primary bacteremia is the 2nd common pattern of infection due to S. paucimobilis following nosocomial infection. Kuar et al. [21] and Bayram et al. [26] showed that primary bacteremia was the commonest presentation in community acquired cases. Bacteremia is defined as either a primary or secondary process. In primary bacteremia, bacteria have been directly introduced into the bloodstream. Injectable drug use may lead to primary bacteremia. In the hospital setting, use of blood vessel catheters contaminated with bacteria may also lead to primary bacteremia. Secondary bacteremia occurs when bacteria have entered the body at another site, such as the cuts in the skin, or mucous membranes of the lungs, mouth or intestine, and bladder or genitals. Furthermore, most patients were either immunocompromised or had underlying diseases. In this case report, patient had 10-year history of diabetes with history of a previous disk surgery. Hence, the infection could have either been nosocomial or community acquired. Incidence of PS ranges from 0.6% to 3.7% after discectomy [27, 28]. There has been only one case report on PS in a patient with bacteremia with positive cultures for S. paucimobilis [16]. Pathogens can reach spinal column by 3 routes: Hematogenous, direct inoculation, or spread from contiguous tissues. Hematogenous arterial route is commonest and it allows seeding of bacilli from distant locations in the spine [16]. In this patient, pathogen could have probably reached through hematogenous route or direct external inoculation considering the patient has a history of a previous disk surgery, leading to PS. Diabetes mellitus is the predominant risk factor. Other predisposing factors are old age, immunocompromised status, injectable drug abuse, renal dysfunction, liver failure, and revision surgery [21]. Indications for surgery in PS include compression of neural elements, instability caused by severe destruction of bony elements, high grade kyphosis, or failure of medical treatment [29, 30, 31]. Some also advocate surgery in the presence of intractable pain [31, 32, 33]. Most authors consider the epidural abscess as indication for surgical intervention, even in neurologically intact patients [34]. This patient had intractable pain with inability to walk, sit, or stand along with epidural, pre, and para vertebral abscesses, which warranted surgical decompression and fusion. PS has lesser osteogenic potential to induce spontaneous fusion than tuberculous spondylodiscitis [35]. Hence, it needs osteogenic material to enhance fusion. The use of autologous interbody graft in PS is well accepted [36, 37, 38, 39, 40]. Hence, we too used a tricortical autograft from iliac crests along with cancellous graft in a PEEK cage as there was extensive bony destruction and tricortical graft alone would not have provided adequate stability. Follow-up radiographs have shown good fusion with good clinical recovery of patient which further proves that surgical decompression and spinal fusion is a good treatment option for PS. No standardized methods presently exist for determining sensitive antibiotics for S. paucimobilis. The bacillus has shown resistance to penicillin and 1st generation cephalosporin due to chromosomal beta-lactamase production [16, 41, 42]. It shows variable sensitivity to 3rd generation cephalosporin and fluoroquinolones [16, 41, 42, 43]. Bayram et al. [26] reported carbapenems to be the maximally effective drug. In a recent case report by Puca et al., [16] on PS at L2-3 in a immunocompetent patient, the organism was susceptible to gentamicin, amikacin, ciprofloxacin, moxifloxacin, imipenem, trimethoprim/sulfamethoxazole, ceftazidime, and cefepime. This case was managed conservatively with imipenem plus ciprofloxacin for 21 days followed by oral therapy for another 4 weeks with ciprofloxacin and trimethoprim/sulfamethoxazole, and responded well. In our case, the organism was found to be susceptible to ceftazidime, cefoperazone, cefepime, linezolid, imipenem, piperacillin, and tazobactam with resistance for penicillin and amoxicillin. Since linezolid is a potent antibiotic available in both IV and oral form, we started the patient on the same and the patient improved clinically. The antibiotic sensitivity of S. paucimobilis differs in all studies published previously. Therefore, it is vital to find the antibiotic susceptibility and treat patients accordingly as sensitive antimicrobials form the mainstay of treatment.
Although S. paucimobilis is an uncommon nosocomial infection, it should be kept in mind as a cause, considering its ubiquitous presence in environment, especially in immunocompromised patients and those with co-morbidities. S. paucimobilis, though has low virulence, is an emerging pathogen that should be dealt with cautiously. PS with bony destruction and instability, and an isolated pathogen, can be treated successfully with surgical debridement and fusion and sensitive antibiotics.
Our case report reiterates the importance of culture as unusual organisms may be isolated and appropriate antibiotics form the mainstay of treatment.
References
- 1.Dervisoglu E, Kalender B, Meric M, Sengul E. Sphingomonas paucimobilis peritonitis: A case report and literature review. Perit Dial Int 2008;28:547-50. [Google Scholar]
- 2.Tai ML, Velayuthan RD. Sphingomonas paucimobilis: An unusual cause of meningitis-case report. Neurol Med Chir (Tokyo) 2014;54:337-40. [Google Scholar]
- 3.Adley CC, Pembroke JT, Ryan MP. Ralstonia pickettii: A persistent gram-negative nosocomial infectious organism. J Hosp Infect 2006;62:278-84. [Google Scholar]
- 4.Hsueh PR, Teng LJ, Yang PC. Nosocomial infections caused by Sphingomonas paucimobilis: Clinical features and microbiological characteristics. Clin Infect Dis 1998;26:676-81. [Google Scholar]
- 5.Casadevall A, Freundlich LF, Pirofski L. Septic shock caused by Pseudomonas paucimobilis. Clin Infect Dis 1992;14:784. [Google Scholar]
- 6.Crane LR, Palutke WA, Tagle LC. Outbreak of Pseudomonas paucimobilis in an intensive care facility. JAMA 1981;246:985-7. [Google Scholar]
- 7.Brunet S, Domingo-Albos A, Martino R, Salazar R, Sureda A, Subira M. Catheter-related bacteremia due to Pseudomonas paucimobilis in neutropenic cancer patients: Report of two cases. Clin Infect Dis 1995;20:1573-4. [Google Scholar]
- 8.Decker CF, Hawkins RE, Simon GL. Infections with Pseudomonas paucimobilis. Clin Infect Dis 1992;14:783-4. [Google Scholar]
- 9.Bullas J, Hajiroussou V, Holmes B, Pinning CA. Meningitis caused by Pseudomonas paucimobilis. J Clin Pathol 1979;32:953-5. [Google Scholar]
- 10.Dratwa M, Glupczynski Y, Hansen W. Pseudomonas paucimobilis peritonitis in patients treated by peritoneal dialysis. J Clin Microbiol 1984;20:1225-6. [Google Scholar]
- 11.Balk RA. Severe sepsis and septic shock. Definitions, epidemiology, and clinical manifestations. Crit Care Clin 2000;16:179-92. [Google Scholar]
- 12.Perola O, Nousiainen T, Suomalainen S, Aukee S, Karkkainen UM. Recurrent Sphingomonas paucimobilis-bacteraemia associated with a multi-bacterial water-borne epidemic among neutropenic patients. J Hosp Infect 2002;50:196-201. [Google Scholar]
- 13.Araújo O, Vidal F, Tapiol J, Richart C. Osteomyelitis caused by Sphingomonas paucimobilis. Enferm Infecc Microbiol Clin 2000;18:247. [Google Scholar]
- 14.White DC, Sutton SD, Ringelberg DB. The genus Sphingomonas: Physiology and ecology. Curr Opin Biotechnol 1996;7:301-6. [Google Scholar]
- 15.Charity RM, Foukas AF. Osteomyelitis and secondary septic arthritis caused by Sphingomonas paucimobilis. Infection 2005;33:93-5. [Google Scholar]
- 16.Puca E, Harxhi A, Mehmeti J, Rroji A, Huti G, Jata B, et al. Sphingomonas paucimobilis from blood stream infection to spondylodiscitis. Clin Microbiol 2015;4:203. [Google Scholar]
- 17.Gusman V, Medic D, Jelesic Z, Ukropina MM. Sphingomonas paucimobilis as a biofilm producer. Arch Biol Sci Belgrade 2012;64:1327-31. [Google Scholar]
- 18.Holmes B, Owen RJ, Evans A, Malnick H, Willcox WR. Pseudomonas paucimobilis, a new species isolated from human clinicalspecimens, the hospital environment, and other sources. Int J Syst Bacteriol 1977;27:133-46. [Google Scholar]
- 19.Yabuuchi E, Yano I, Oyaizu H, Hashimoto Y, Ezaki T, Yamamoto H. Proposals of Sphingomonas paucimobilis gen. nov. and comb. nov., Sphingomonas parapaucimobilis sp. nov., Sphingomonas yanoikuyae sp. nov., Sphingomonas adhaesiva sp. nov., Sphingomonas capsulata comb. nov., and two genospecies of the genus Sphingomonas. Microbiol Immunol 1990;34:99-119. [Google Scholar]
- 20.Ã-zdemir M, Demircili ME, Pekcan S, Taşbent FE, Feyzioğlu B, Pirinç S, et al. A rare cause of bacteremia in a pediatric patient with Down syndrome: Sphingomonas paucimobilis. Int J Med Sci 2011;8:537-9. [Google Scholar]
- 21.Kuar WK, Toh HS, Tay HT, Weng TC, Tang HJ, Tan CK, et al. Risk factors associated with Sphingomonas paucimobilis infection. J Microbiol Immunol Infect 2011;44:289-95. [Google Scholar]
- 22.Maragakis LL, Chaiwarith R, Srinivasan A, Torriani FJ, Avdic E, Lee A, et al. Sphingomonas paucimobilis bloodstream infections associated with contaminated intravenous fentanyl. Emerg Infect Dis 2009;15:12-8. [Google Scholar]
- 23.Reina J, Bassa A, Llompart I, Portela D, Borrell N. Infections with Pseudomonas paucimobilis: Report of four cases and review. Rev Infect Dis 1991;13:1072-6. [Google Scholar]
- 24.Kucukbayrak A, Demirli K, Ozdemir D, Kucukbayrak ZS, Hakyemez IN. Primary bacteremia associated With Sphingomonas paucimobilis during the late period in a patient with ventriculoperitoneal shunt after neurosurgery with literature review. Neurosurg Q 2012;22:38-40. [Google Scholar]
- 25.Cheong HS, Moon SY, WiYM. Clinical features and treatment outcomes of infections caused by Sphingomonas paucimobilis. Infect Control Hosp Epidemiol 2008;29:990-2. [Google Scholar]
- 26.Bayram N, Devrim I, Apa H, Gülfidan G, Türkyılmaz HN, Günay I. Sphingomonas paucimobilis Infections in children: 24 Case reports. Mediterr J Hematol Infect Dis 2013;5:e2013040. [Google Scholar]
- 27.Rohde V, Meyer B, Schaller C, Hassler WE. Spondylodiscitis after lumbar discectomy. Incidence and a proposal for prophylaxis. Spine (Phila Pa 1976) 1998;23:615-20. [Google Scholar]
- 28.Curtin JA, Horwitz NH. Prophylactic antibiotics and wound infections following laminectomy for lumbar disc herniation. J Neurosurg 1975;43:727-31. [Google Scholar]
- 29.Sobottke R, Seifert H, Fatkenheuer G. Current diagnosis and treatment of spondylodiscitis. Dtsch Arztebl Int 2008;105:181-7. [Google Scholar]
- 30.Hsieh PC, Wienecke RJ, O’Shaughnessy BA. Surgical strategies for vertebral osteomyelitis and epidural abscess. Neurosurg Focus 2004;17:E4. [Google Scholar]
- 31.Chen WH, Jiang LS, Dai LY. Surgical treatment of pyogenic vertebral osteomyelitis with spinal instrumentation. Eur Spine J 2007;16:1307-16. [Google Scholar]
- 32.Rezai AR, Woo HH, Errico TJ. Contemporary management of spinal osteomyelitis. Neurosurgery 1999;44:1018-25. [Google Scholar]
- 33.Hee HT, Majd ME, Holt RT. Better treatment of vertebral osteomyelitis using posterior stabilization and titanium mesh cages. J Spinal Disord Tech 2002;15:149-56; discussion 156. [Google Scholar]
- 34.Darouiche RO. Spinal epidural abscess. N Engl J Med 2006;355:2012-20. [Google Scholar]
- 35.Moon MS, Moon YW, Moon JL, Kim SS, Sun DH. Conservative treatment of tuberculosis of the lumbar and lumbosacral spine. Clin Orthop Relat Res 2002;398:40-9. [Google Scholar]
- 36.Calderone RR, Larsen JM. Overview and classification of spinal infections. Orthop Clin North Am 1996;27:1-8. [Google Scholar]
- 37.Carragee EJ. Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am 1997;79:874-80. [Google Scholar]
- 38.Rigamonti D, Liem L, Sampath P. Spinal epidural abscess: Contemporary trends in etiology, evaluation, and management. Surg Neurol 1999;52:189-97. [Google Scholar]
- 39.Fang D, Cheung KM, dos Remedios ID, Lee YK, Leong JC. Pyogenic vertebral osteomyelitis: Treatment by anterior spinal debridement and fusion. J Spinal Disord 1994;7:173-80. [Google Scholar]
- 40.Jackson JW, Jeremiah JD, Kemp HB. Anterior fusion of the spine for infective lesions in adults. J Bone Joint Surg Br 1973;55:715-34. [Google Scholar]
- 41.Morrison AJ Jr., Shulman JA. Community-acquired bloodstream infection caused by Pseudomonas paucimobilis: Case report and review of the literature. J Clin Microbiol 1986;24:853-5. [Google Scholar]
- 42.Smalley DL, Hansen VR, Baselski VS. Susceptibility of Pseudomonas paucimobilis to 24 antimicrobial agents. Antimicrob Agents Chemother 1983;23:161-2. [Google Scholar]