The definitive management for APL and EPB hemangioma was excision biopsy in toto which provided better functional results in our patient.
Dr. Madhan Jeyaraman, Department of Orthopedics, School of Medical Sciences and Research, Sharda University, Greater Noida – 201 306, Uttar Pradesh, India. E-mail: madhanjeyaraman@gmail.com
Introduction: Although upper extremity intramuscular hemangioma is a rare clinical entity, it poses considerable morbidity in the functional needs of an individual. The diagnosis of intramuscular hemangioma poses a diagnostic glitch. The combined radiological and histopathological assessment provides a complete understanding and diagnosis for the same. Every tumor follows an individualized protocol for its management.
Case Report: A 15-year-old female presented with swelling over dorsal aspect of distal 1/3rd right forearm, which was 3 cm away from the articular surface of the right wrist from the past 5 years. There was no history of trauma or infection over the right wrist. Finkelstein’s test was negative, which rule out de Quervain’s tenosynovitis. The movements of the right wrist were unrestricted without any distal neurovascular deficit. MRI of her right wrist and hand suggested the presence of low flow vascular malformation within the musculotendinous junction of APL and EPB muscles. The patient underwent excision biopsy of the hemangiomatous lesion in toto without any neurological complications. No recurrence was noted in the follow-up period for 6 months.
Conclusion: Being a benign vascular tumor, MRI provides the gateway to diagnose intramuscular hemangioma for early intervention to provide better functional results. The choice of definitive treatment for APL and EPB hemangioma was excision biopsy in toto which provided better functional results in our patient.
Keywords: Hemangioma, intramuscular, abductor pollicis longus, extensor pollicis brevis, MRI, excision biopsy.
Hemangiomas, the most common benign tumor of vascular channels, are of various types, namely, capillary, cavernous, or mixed varieties [1, 2]. Capillary hemangioma occurs in the superficial plane whereas cavernous hemangioma occurs both in superficial and deep planes of the body tissues [3, 4, 5, 6]. Since the pathogenesis of hemangioma is not understood, the management of the tumor remains controversial. Intramuscular hemangioma is a non-malignant, hamartomatous neoplasm that remains unrecognized for a longer period [7, 8]. Intramuscular hemangiomas are usually invasive tumors that manifest in either muscle, bone, or viscera [6, 8]. These hemangiomas may be engorged with blood during activities causing pain and swelling. Lower extremity (45%) hemangioma is more common in occurrence than in the upper extremity (27%) [2, 9]. Superficial skin changes are often not present but they present with calcifications or phleboliths which are diagnostic of cavernous type of hemangioma [3, 4, 10, 11]. Hemangioma of musculotendinous junction of APL and EPB has not been reported yet in literature. We present a case of APL and EPB hemangioma and its management in this case report.
A 15-year-old female presented with the swelling over distal 1/3rd right forearm from the past 5 years, as shown in (Fig. 1). The swelling was insidious in onset and slowly increasing in size. The swelling was associated with mild pain which was non-radiating to the right forearm or arm but localized to the dorsal aspect of distal 1/3rd of the right forearm which was 3 cm away from the articular surface of the right wrist. There was no history of injury, infection, sinus, or other swellings on her forearm. On examination, there was a diffuse, oval-shaped, solitary swelling measuring about 3.5 × 2.5 cm with its surface smooth, edges being indistinct, non-pulsatile, non-fluctuant, non-transilluminant, non-compressible, and non-reducible present over distal 1/3rd of radial side of the dorsal aspect of the right forearm. The swelling was non-adherent to the skin and underlying structures. No muscle wasting was present. There were no arterial bruits and venous hum on auscultation. The distal neurovascular status was intact. Finkelstein’s test was negative, which rule out de Quervain’s tenosynovitis. The range of motions of the right wrist was full and free without any pain. There was no regional lymphadenopathy. A wide range of differential diagnosis of intramuscular lipoma, muscular fibroadenoma, hemangioma, angiofibroma, lymphangioma, neurofibroma, nodular fasciitis, and peripheral nerve sheath tumors was considered.
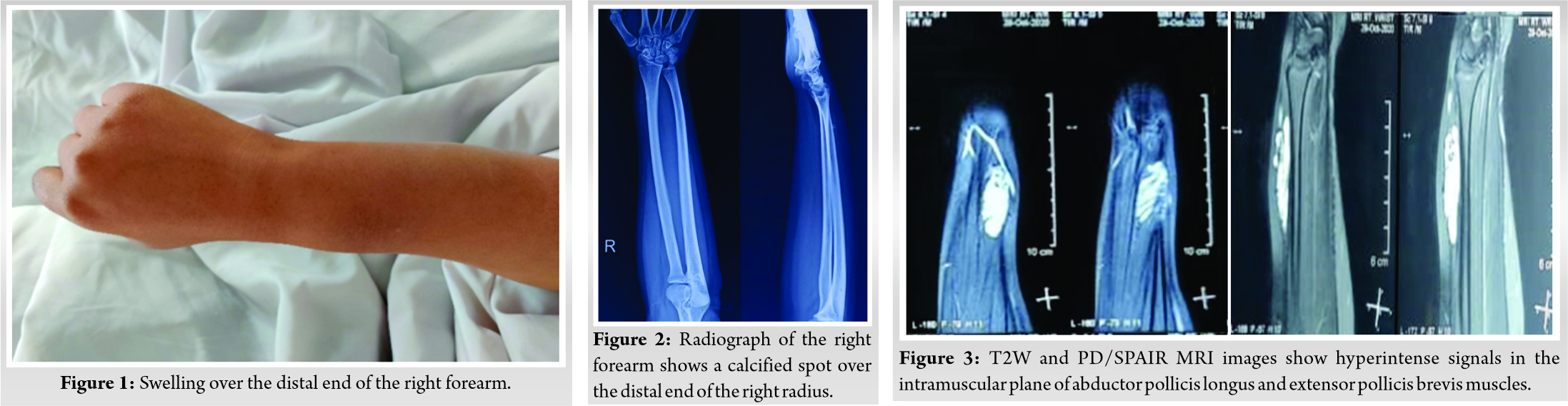
Plain radiograph of the right wrist revealed a calcified spot in the distal end of the right radius, as shown in (Fig. 2). Ultrasound of the right wrist revealed numerous irregular hypoechoic areas suggestive of low flow vascular tumor. MRI of the right wrist revealed a lobulated lesion (showing T2 and PD/SPAIR hyperintense signals) in the intramuscular plane measuring about 1.8 × 1.2 × 4.2 cm at the radial side in the extensor aspect involving the muscles of abductor pollicis longus and extensor pollicis brevis, as shown in (Fig. 3). Under general anesthesia, an excisional biopsy of the mass, which was arising from the musculotendinous junction of APL and EPB, was done with an uneventful post-operative period, as shown in (Fig. 4). Grossly, it was partially circumscribed soft-tissue mass measuring 5.5 × 2.5 cm. The cut section of the mass showed numerous vessels of variable sizes containing thrombus and hemorrhage. Histopathology of the biopsied muscle revealed an infiltrating tumor composed of variable sized vascular spaces with a predominance of larger sized blood vessels, which are lined by endothelial cells and show RBCs in the lumen. Irregular thin-walled vascular channels were infiltrating within skeletal muscle bundles and fat, as shown in (Fig. 5). The surrounding area showed mature fatty tissues which are suggestive of cavernous hemangioma. The patient has been followed up at regular intervals for 6 months postoperatively during which the patient showed a full range of movements of the thumb. No recurrence was noted during the follow-up period. The patient was still under follow-up.

Hemangiomas contribute to 7% of all soft-tissue tumors, whereas intramuscular hemangiomas are 0.8% of all hemangiomas [2]. Hemangiomas are the hamartomatous proliferation of vascular endothelial cells, hyperplastic endothelium, increased cellular mitogenicity, or ectatic blood channels [12, 13]. Hemangiomas are of capillary, cavernous, or mixed variety. Female preponderance (3–5:1) was seen with the hemangiomas and the most common occurrence were reported before the age of 30 years (90%) [2, 14, 15, 16]. Occasionally, hemangiomas were reported in the deeper soft tissues. They express GLUT-1 receptors which are RBC-dependent glucose transporter protein [17, 18, 19]. Intramuscular hemangioma has no relationship with traumatic etiology [10, 20]. Upper extremity hemangiomas (triceps, biceps, FDS, FDP, and pronator quadratus) contribute to 27% of intramuscular hemangioma [2]. Kayias et al. reported a case of hemangioma in extensor pollicis brevis with periosteal reaction in radius [21]. Histologically, Kayias et al. proposed a classification for intramuscular hemangioma, namely, (a) without periosteal reaction, (b) with periosteal reaction, and (c) with bone and bone marrow involvement [21]. No case was reported with the detailed reports of hemangioma in abductor pollicis longus (APL) and extensor pollicis brevis (EPB). Intramuscular hemangioma presents as a slow-growing mass with compressibility and without pulsations or bruits due to the presence of minor vascular malformations [16, 22, 23, 24]. Intramuscular hemangioma poses a major diagnostic challenge [7, 11, 25]. In our case, we found the hemangioma being situated in the flare of musculotendinous portions of APL and EPB without any restrictions of wrist and thumb movements. Radiograph of the right wrist revealed the presence of phleboliths in the distal end of the right radius with soft-tissue swelling. USG of the right wrist showed low flow vascular malformations in the musculotendinous junction of the right APL and EPB. MRI, being the gold standard diagnostic of choice for hemangioma, revealed lobulated lesion (T2 and PD/SPAIR hyperintense signals) in the musculotendinous portion of APL and EPB measuring 1.8 × 1.2 × 4.2 cm at the radial side with few areas of T2 hypointense signals indicating the presence of phleboliths. There was no scalloping effect noticed in the distal end of the right radius. Our case findings are consistent with the reported intramuscular hemangioma. No features of malignancy were observed in the case. The well-defined intramuscular appearance of the hemangioma revealed resectability and hence confirming a benign lesion of vascular origin [2, 7, 10]. Intramuscular hemangioma produces a scalloping effect in the neighboring bone as mentioned by Kumar et al. [2] and Ly et al. [26]. In our case, we did not observe any scalloping effect or a cortical breach in the neighboring bone. The management of intramuscular hemangioma depends on the tumor location, size, extent, behavior, accessibility, resectability, and cosmetic indications [10, 27]. Depending on the size, the management differ in terms of conservative treatment modalities (spontaneous regression [28], interferon-alpha [29], imiquimod [30], cyclophosphamide [31], and vincristine [32]), minimally invasive methods (radioisotope agents such as strontium-90 [33], sclerotherapy [34], cryotherapy [35], radiation therapy [36], carbon dioxide snow [37], argon laser [38], and Nd:YAG laser [39]), surgical options (ligation of feeding vessels [7] and excision biopsy in toto [2]), and combination therapy [40, 41, 42, 43, 44, 45]. We instituted excision biopsy in toto to provide a better functional outcome. It is reported that inadequate excision leads to a recurrence rate between 20% and 60% [2, 26, 46, 47]. No recurrence was reported in our case in the past 6 months follow-up. To date, we found a few published pieces of literature on EPB hemangioma which is tabulated in Table 1. One report on APL, EPB, and supinator hemangioma was reported by Yuh et al. but the surgical and prognostic details were found missing in the literature [48]. According to Kayias et al. classification of intramuscular hemangioma, our case belongs to type 1 intramuscular hemangioma without periosteal reaction [21].
Being a benign vascular tumor, MRI provides the gateway to diagnose intramuscular hemangioma for early intervention to provide better functional results. The choice of definitive treatment for APL and EPB hemangioma was excision biopsy in toto which provided better functional results in our patient.
Being a benign vascular tumor, MRI provides the gateway to diagnose intramuscular hemangioma for early intervention to provide better functional results.
References
- 1.George A, Mani V, Noufal A. Update on the classification of hemangioma. J Oral Maxillofac Pathol 2014;18:S117-20. [Google Scholar]
- 2.Kumar R, Ranjan R, Jeyaraman M, Chaudhary D, Arora A, Kumar S. Pronator quadratus hemangioma (PQH): A rare case report and review. Indian J Orthop 2021. (Published online) [Google Scholar]
- 3.Pourbagher A, Pourbagher MA, Karan B, Ozkoc G. MRI manifestations of soft-tissue haemangiomas and accompanying reactive bone changes. Br J Radiol 2011;84:1100-8. [Google Scholar]
- 4.Olsen KI, Stacy GS, Montag A. Soft-tissue cavernous hemangioma. Radiographics. 2004;24:849-54. [Google Scholar]
- 5.Goto T, Kojima T, Iijima T, Yokokura S, Kawano H, Yamamoto A, et al. Soft-tissue haemangioma and periosteal new bone formation on the neighbouring bone. Arch Orthop Trauma Surg 2001;121:549-53. [Google Scholar]
- 6.Allen PW, Enzinger FM. Hemangioma of skeletal muscle. An analysis of 89 cases. Cancer 1972;29:8-22. [Google Scholar]
- 7.Lakshmi KC, Sankarapandiyan S, Mohanarangam VS. Intramuscular haemangioma with diagnostic challenge: A cause for strange pain in the masseter muscle. Case Rep Dent 2014;2014:e285834. [Google Scholar]
- 8.Nayak S, Shenoy A. Intra-muscular hemangioma: A review. J Orofac Sci 2014;6:2. [Google Scholar]
- 9.Jacobs BJ, Anzarut A, Imbriglia JE. Vascular anomalies of the upper extremity. J Hand Surg 2010;35:1703-9. [Google Scholar]
- 10.Wierzbicki JM, Henderson JH, Scarborough MT, Bush CH, Reith JD, Clugston JR. Intramuscular Hemangiomas. Sports Health 2013;5:448-54. [Google Scholar]
- 11.Pattamapaspong N, Peh WC, Shek TW. Imaging of intramuscular haemangiomas of the extremities. Singapore Med J 2020;61:122-8. [Google Scholar]
- 12.Bruder E, Alaggio R, Kozakewich HP, Jundt G, Dehner LP, Coffin CM. Vascular and perivascular lesions of skin and soft tissues in children and adolescents. Pediatr Dev Pathol 2012;15 Suppl 1:26-61. [Google Scholar]
- 13.Dhiman NK, Jaiswara C, Kumar N, Patne SC, Pandey A, Verma V. Central cavernous hemangioma of mandible: Case report and review of literature. Natl J Maxillofac Surg 2015;6(2):209-213. [Google Scholar]
- 14.Patten DK, Wani Z, Kamineni S. Intramuscular cavernous haemangioma of the triceps. Int J Surg Case Rep 2011;2:86-9. [Google Scholar]
- 15.Rastogi A, Srivatsava N, Varun V, Agarwal S. Intramuscular Cavernous Hemangioma of the Triceps Muscle. Ind J Vas Endovas Surg 2015;2(3):122-124. [Google Scholar]
- 16.Patnaik S, Kumar P, Nayak B, Mohapatra N. Intramuscular arteriovenous hemangioma of thigh: A case report and review of literature. J Orthop Case Rep 2016;6:20-3. [Google Scholar]
- 17.Leon-Villapalos J, Wolfe K, Kangesu L. GLUT-1: An extra diagnostic tool to differentiate between haemangiomas and vascular malformations. Br J Plast Surg 2005;58:348-52. [Google Scholar]
- 18.da Silva Filho TJ, de Oliveira DH, de Souza Moura I, da Silva Medeiros LK, Gonzaga AK, Brasil VL, et al. Importance of GLUT1 in differential diagnosis of vascular anomalies. J Vasc Bras 2015;14:168-76. [Google Scholar]
- 19.North PE, Waner M, Mizeracki A, Mihm MC. GLUT1: A newly discovered immunohistochemical marker for juvenile hemangiomas. Hum Pathol 2000;31:11-22. [Google Scholar]
- 20.Niempoog S, Pholsawatchai W. Intramuscular hemangioma of the forearm with flexion contracture. Case Rep Orthop. 2019;2019:e6024039. [Google Scholar]
- 21.Kayias EH, Drosos GI, Kazakos KI, Iatrou C, Blatsoukas KS, Verettas DA. Intramuscular haemangioma of the extensor pollicis brevis muscle with periosteal reaction of the radius: A case report and review of the literature. J Int Med Res 2007;35:724-30. [Google Scholar]
- 22.Park JW, Kim CH, Moon CW. Intramuscular hemangioma in buccal cheek: A case report. J Korean Assoc Oral Maxillofac Surg 2017;43:262-6. [Google Scholar]
- 23.Liu Y, Li R, Liu Z, Wang S, Lu L. Intramuscular hemangioma within the biceps brachii causing the limitations of elbow extension and forearm pronation. Medicine (Baltimore) 2019;98:e14343. [Google Scholar]
- 24.Kiran KR, Babu TV, Babu SS, Deepti K. Skeletal muscle haemangioma: A cause for chronic pain about the knee: A case report. Case Rep Orthop 2012;2012:e452651. [Google Scholar]
- 25.Wu JL, Wu CC, Wang SJ, Chen YJ, Huang GS, Wu SS. Imaging strategies in intramuscular haemangiomas: An analysis of 20 cases. Int Orthop 2007;31:569-75. [Google Scholar]
- 26.Ly JQ, Sanders TG, Mulloy JP, Soares GM, Beall DP, Parsons TW, et al. Osseous change adjacent to soft-tissue hemangiomas of the extremities: Correlation with lesion size and proximity to bone. Am J Roentgenol 2003;180:1695-700. [Google Scholar]
- 27.Lahrach K, Abdulrazak S, Marzouki A, Boutayeb F. Surgical management of intramuscular hemangioma of the foot: A case report. Patient Saf Surg 2019;13:17. [Google Scholar]
- 28.Priestnall SL, De Bellis F, Bond R, Alony-Gilboa Y, Summers BA. Spontaneous regression of congenital cutaneous hemangiomas in a calf. Vet Pathol 2010;47:343-5. [Google Scholar]
- 29.Ricketts RR, Hatley RM, Corden BJ, Sabio H, Howell CG. Interferon-alpha-2a for the treatment of complex hemangiomas of infancy and childhood. Ann Surg 1994;219:605-14. [Google Scholar]
- 30.Mao X, Wang J, Yan J. Topical imiquimod treatment of cutaneous vascular disorders in pediatric patients: Clinical evaluation on the efficacy and safety. J Zhejiang Univ Sci B 2012;13:745-50. [Google Scholar]
- 31.Wilson MW, Hoehn ME, Haik BG, Rieman M, Reiss U. Low-dose cyclophosphamide and interferon alfa 2a for the treatment of capillary hemangioma of the orbit. Ophthalmology 2007;114:1007-11. [Google Scholar]
- 32.Wasserman JD, Mahant S, Carcao M, Perlman K, Pope E. Vincristine for successful treatment of steroid-dependent infantile hemangiomas. Pediatrics 2015;135:e1501-5. [Google Scholar]
- 33.Sklaroff DM. Treatment of hemangiomas with the strontium-90 beta-ray applicator. Radiology 1957;68:87-9. [Google Scholar]
- 34.Silva VA, Lima NL, Mesquita AT, da Silveira EM, Verli FD, de Miranda JL, et al. Intramuscular hemangioma in lip treated with sclerotherapy and surgery. Case Rep Dent 2011;2011:302451. [Google Scholar]
- 35.Carabin J, Bouhamama A, Vaz G, Cuinet M, Ricoeur A, Thibaut A, et al. Percutaneous cryoablation of symptomatic intramuscular venous malformation. J Vasc Interv Radiol 2020;31:558-63.e3. [Google Scholar]
- 36.Park S, Yoon SM, Lee S, Park JH, Song SY, Lee SW, et al. Role of fractionated radiotherapy in patients with hemangioma of the cavernous sinus. Radiat Oncol J 2017;35:268-73. [Google Scholar]
- 37.Parvathidevi MK, Koppal S, Rukmangada T, Byatnal AR. Management of haemangioma with sclerosing agent: a case report. BMJ Case Rep 2013;2013:bcr2013200660. [Google Scholar]
- 38.Apfelberg DB, Greene RA, Maser MR, Lash H, Rivers JL, Laub DR. Results of argon laser exposure of capillary hemangiomas of infancy–preliminary report. Plast Reconstr Surg 1981;67:188-93. [Google Scholar]
- 39.Vlachakis I, Gardikis S, Michailoudi E, Charissis G. Treatment of hemangiomas in children using a Nd:YAG laser in conjunction with ice cooling of the epidermis: techniques and results. BMC Pediatr 2003;3:2. [Google Scholar]
- 40.Tang P, Hornicek FJ, Gebhardt MC, Cates J, Mankin HJ. Surgical treatment of hemangiomas of soft tissue. Clin Orthop 2002;399:205-10. [Google Scholar]
- 41.Wild AT, Raab P, Krauspe R. Hemangioma of skeletal muscle. Arch Orthop Trauma Surg 2000;120:139-43. [Google Scholar]
- 42.Mitsionis GI, Pakos EE, Kosta P, Batistatou A, Beris A. Intramuscular hemangioma of the foot: A case report and review of the literature. Foot Ankle Surg 2010;16:e27-9. [Google Scholar]
- 43.Mencke HJ, Zilkens J, Bigalke KH, Ammon J. The problem of intramuscular haemangioma. Arch Orthop Trauma Surg 1982;100:243-7. [Google Scholar]
- 44.McNeill TW, Chan GE, Capek V, Ray RD. The value of angiography in the surgical management of deep hemangiomas. Clin Orthop 1974;101:176-81. [Google Scholar]
- 45.Tiwari P, Kaur H, Jha V, Bansal K. Intramuscular Vascular Malformation in Triceps: A Case Report with Literature Review. Ind J Vas Endovas Surg 2020;7(3):277-280. [Google Scholar]
- 46.Bella GP, Manivel JC, Thompson RC, Clohisy DR, Cheng EY. Intramuscular hemangioma: Recurrence risk related to surgical margins. Clin Orthop Relat Res 2007;459:186-91. [Google Scholar]
- 47.DeGiovanni JC, Simmonds J, Lang-Orsini M, Lee A. Recurrent intramuscular hemangioma (infiltrating angiolipoma) of the lower lip: A case report and review of the literature. Ear Nose Throat J 2020;2020:145561320957759. [Google Scholar]
- 48.Yuh WT, Kathol MH, Sein MA, Ehara S, Chiu L. Hemangiomas of skeletal muscle: MR findings in five patients. AJR Am J Roentgenol 1987;149:765-8. [Google Scholar]
- 49.Waddell GF. A haemangioma involving tendons. J Bone Joint Surg Br 1967;49:138-41. [Google Scholar]
- 50.Weinzweig J, Watson HK, Wiener BD, Genter BE. Hemangioma of the extensor pollicis brevis in the first dorsal compartment: An unusual cause of bilateral de Quervain’s disease. J Hand Surg 1996;21:256-8. [Google Scholar]











