Magnetically controlled growing rods can undergo complete magnet fracture in the absence of clinical or radiological features.
Dr. Conor S Jones,
Department of Spine, Exeter Spine Unit, Royal Devon and Exeter NHS Found Foundation Trust, Exeter, United Kingdom.
E-mail: Drconorsjones@gmail.com
Introduction: Magnetically controlled growing rods (MCGRs) have been widely adopted in the management of early-onset scoliosis since they were first described in 2012. Recent reports have highlighted concerns around their safety. To date, little is understood about the risk factors and modes of failure in these devices.
Case Report: We report a novel mechanism of device failure in a 14-year-old patient following multiple revisions of MCGRs. Clinically, there was no evidence of device failure and the MCGRs appeared radiologically intact. Explantation analysis revealed multiple compromised/non-functional components. A previously undocumented phenomenon of complete magnet fracture was also seen.
Conclusion: The absence of clinical or radiological features of device failure in this case makes the findings of great concern. Given the relative paucity of high-quality evidence surrounding the use of MCGRs, we support calls for urgent comparative studies and further investigation of risk factors for device failure.
Keywords: Spinal surgery, Scoliosis, Magnetically controlled growing rods, Implant.
Early-onset scoliosis (EOS) is characterized by a lateral curvature of the spine before the age of 10 years. Its management is challenging due to the rapid rate of spinal, thoracic, and pulmonary development at this age. Growing rods are a recognized treatment for progressive curves, they permit guided spinal growth until sufficient skeletal maturity for definitive fusion is reached. Unlike traditional growing rods (TGRs), which require repeated invasive distraction under general anesthesia, magnetically controlled growing rods (MCGRs) contain a telescopic actuator which can be lengthened non-invasively in the outpatient setting. Since they were first described by Cheung et al. in 2012, MCGRs have been adopted as a treatment for EOS worldwide [1]. Early reports of their use have demonstrated promising results in terms of spinal curvature control [1, 2, 3]. More recently, however, concerns have been raised about the long-term effectiveness of MCGRs and potentially high failure rate [4, 5]. Current methods of introducing surgical devices have been the subject of much controversy and concern in recent years [6]. Unlike the pharmaceutical industry, the evaluation and introduction of surgical devices are less tightly regulated. As a result, device failure can go unrecognized by the surgical community until centers have implanted enough devices, which have subsequently failed, begin to report concerns in the published in the literature. This permits the continued use of potentially harmful devices and presents risk to patients. Case reports, therefore, play an important role in the early identification of safety concerns. Here, we report a new mechanism of device failure in a 14-year-old patient following multiple surgical revisions of MCGRs.
An 8-year-old female patient initially underwent treatment with dual construct MCGRs (MAGnetic Expansion Control, Ellipse Technologies Inc., Aliso Viejo, CA, USA [MAGEC]) for idiopathic early-onset scoliosis as described by Cheung et al. (Fig. 1) [1]. Bilateral drive pin fracture, within the actuator portion of the rods, necessitated rod exchange after 38 months in vivo. This mechanism of failure has been reported elsewhere [7].
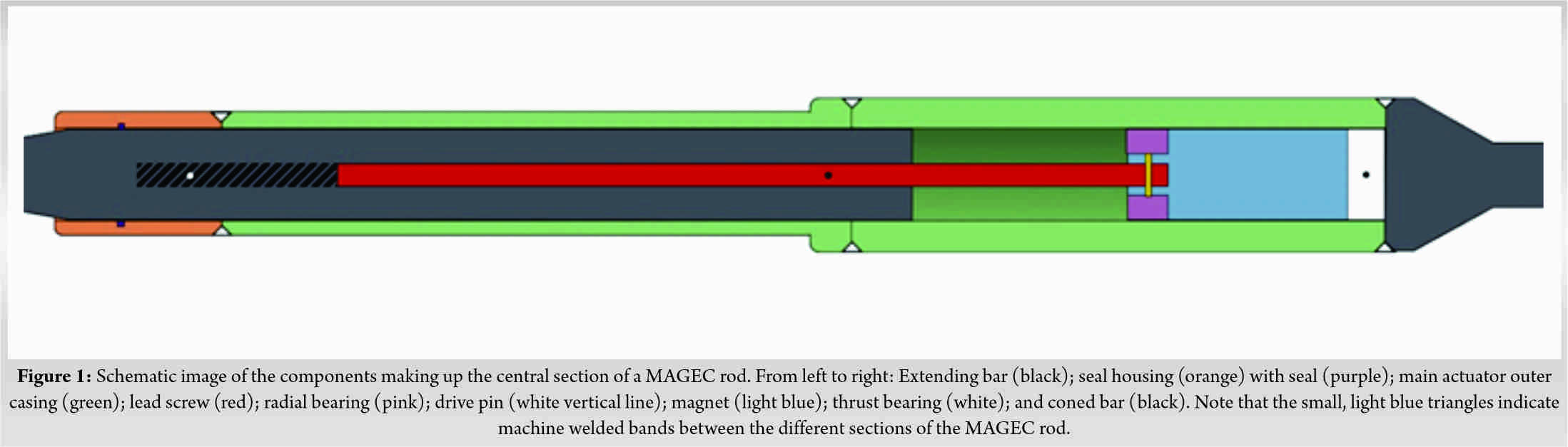
Further revision of the concave rod was required after 21 months, with the patient aged 13 years. Radiologically, failure of the distal end cap to restrain the MCGR during distraction was noted. Following discussion with the manufacturer, the decision was made to exchange the entire MCGR, as opposed to reimplantation, due to the risk of notching and consequent failure of the in situ rod. A 4.5 mm offset rod with 90 mm actuator MAGEC rod (version 1.5) was contoured, tested, and inserted before set screw application and tightening. Distractions took place at 3-month intervals until clunking was heard. Definitive fusion was performed 18 months after the second revision (39 months after the initial revision) when the patient had reached sufficient skeletal maturity. Radiologically, the rods appeared intact and there was no clinical indication of device failure (Fig. 2). Pre-explantation radiographs demonstrated coronal Cobb angle of 42° with good coronal balance.Intraoperatively, extensive metallosis was seen bilaterally. An additional metallosis tract remained at the site of a previously explanted device.
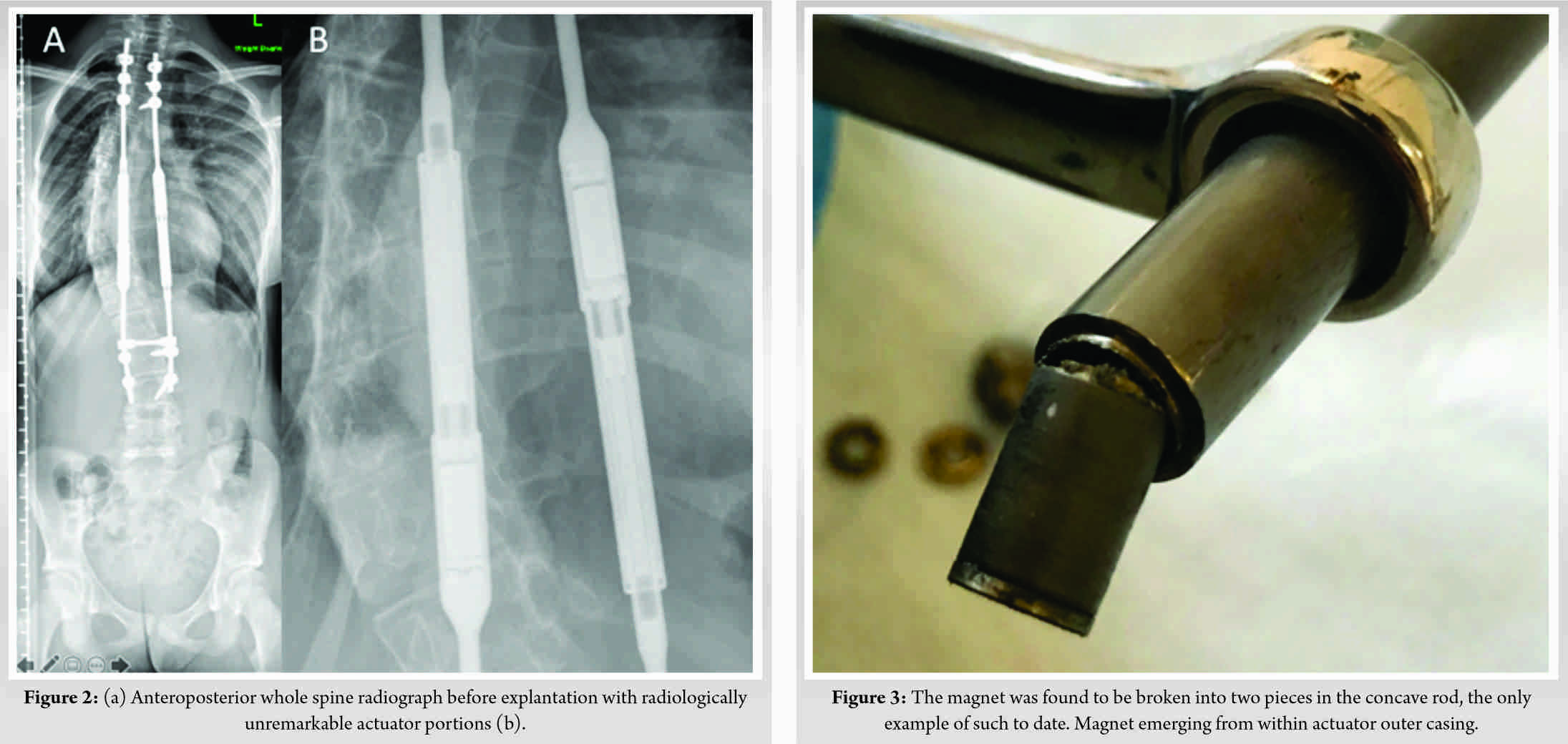
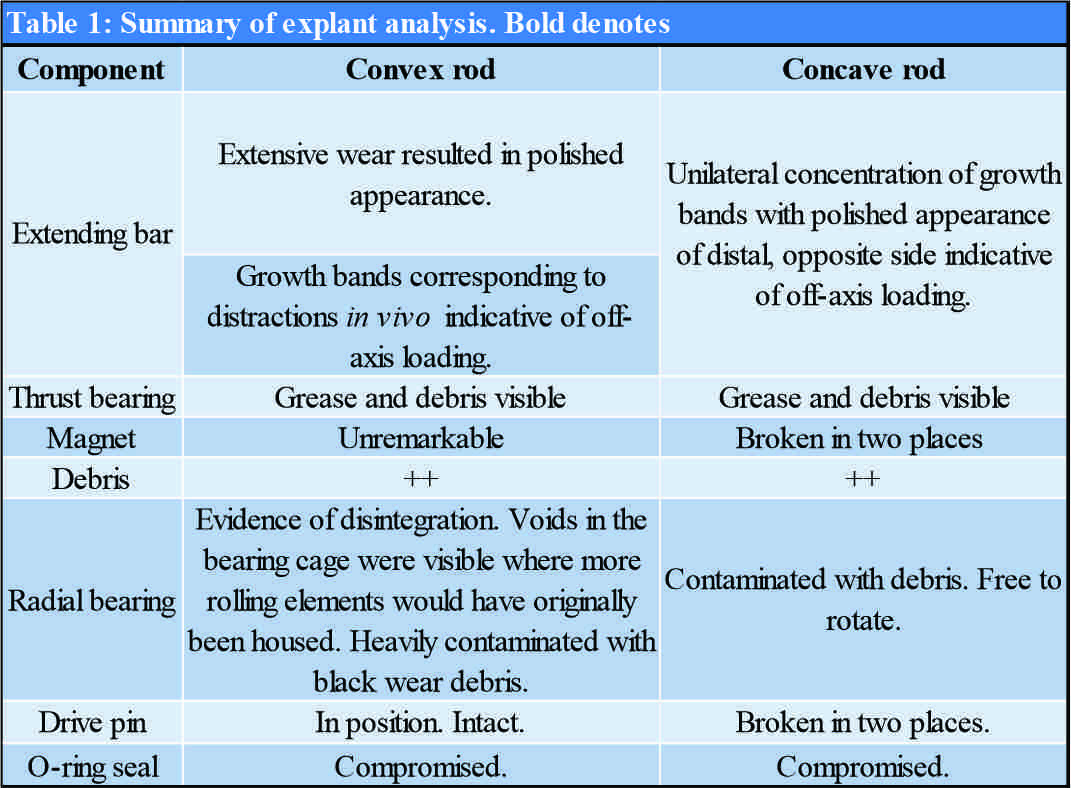
Explant analysis was performed by an independent retrieval center (Newcastle Upon Tyne, United Kingdom). Neither rod was able to produce any force ex vivo when tested with an external remote controller. The actuator casing was cut open to interrogate the internal components. Findings of the explanation analysis are summarized in (Table 1). A previously undocumented phenomenon, complete fracture of the magnet, was identified within the concave MCGR (Fig. 3). Both rods demonstrated compromise of the sealing mechanism (Fig. 4) and extensive accumulation of black debris. The drive pin was fractured in the concave rod only (Fig. 5).

This case adds to the growing body of literature, highlighting the high potential for mechanical failure of MCGRs [8, 9].
The risk of experiencing a complication associated with TGRs increases by 24% with each successive operation and reaches 47% by the seventh procedure [10]. MCGR technology was, therefore, developed to allow non-surgical distraction in the outpatient setting. However, a recent review of studies with minimum 2-year follow-up has demonstrated complication and reoperation rates of 48% and 44%, respectively [4]. Furthermore, the majority of MCGRs are non-functional at the time of removal and rarely distract fully in situ with mean distraction of 22 mm over their lifetime [9].
Surgeons should be aware that catastrophic implant failure of MAGEC rods can occur in the absence of obvious clinical or radiographic findings. The compromised sealing mechanism is thought to enable fluid and material to pass from the rod internals to localized tissues resulting in metallosis [9, 11]. Furthermore, MCGRs have demonstrated greater release of titanium and vanadium ions into the blood than TGRs [12]. The long-term health implications of this remain unknown. To date, over 140 MCGRs have been assessed by the retrieval center in Newcastle upon Tyne [9]. However, the phenomenon of complete magnet fracture had not been seen and, to the best of our knowledge, has not been reported in the literature. Other features outlined in Table 1 are more common. The characteristic wear pattern, damage to the radial bearing, O-ring failure, and drive pin fracture have been reported to occur in 100%, 74%, 53%, and 44% of explanted MCGRs, respectively [9]. These features are indicative of offset loading and bending forces placed on the device. Application of these forces across the magnet itself may have driven its failure through fatigue. The fracture point itself appears to be in the center of the magnet in at a junction in the magnet casing. This surface irregularity may indicate notch sensitivity influencing the magnet fracture. It is understood that single rod constructs should be avoided [13]. However, other risk factors for device failure remain poorly understood. Patient, implant, and operative factors are likely to contribute. The patient in this case was 13 years old at the time of implantation toward the older limit of the implant’s intended use. Manufacturing processes could feasibly have also contributed. MCGRs have undergone multiple iterations since their initial development [8]. In June 2019, the manufacturer of MCGRs released an urgent field safety notice recognizing the issue of fractured internal locking pins in early versions of the device manufactured prior to March 26, 2015. The manufacturer stated that these models are no longer available for sale or implantation [14]. In our case, the concave rod was marked A170213-09. The number, we believe, reveals that this rod was made on February 13, 2017, as part of batch 09. Given the average lifespan of around 35 months, few MCGRs from 2017 have been analyzed to date [9]. We are, therefore, unable comment on whether this was an isolated issue, or whether it could occur in other devices produced at this time. In March 2020, the MAGEC System Model X, a newer modification of the device was withdrawn from the market due to safety concerns [15]. The subsequent increase in use of “older” models further increases the clinical relevance of this issue.
Given the relative paucity of high-quality evidence surrounding the use of MCGRs and implications of device failure, we support calls for urgent comparative studies and further investigation of the risk factors for device failure.
The rapid uptake of MCGRs has not been matched by thorough evaluation of risk factors and mechanisms of device failure. We report a novel mechanism of device failure in a 14-year-old patient involving complete fracture of the magnet component. The absence of clinical or radiological features of device failure in this case makes the findings of great concern. Many patients around the world are currently living with this implant in situ. We support calls for urgent comparative studies and further investigation of risk factors for device failure.
References
- 1.Cheung KM, Cheung JP, Samartzis D, Mak KC, Wong YW, Cheung WY, et al. Magnetically controlled growing rods for severe spinal curvature in young children: A prospective case series. Lancet 2012;379:1967-74. [Google Scholar]
- 2.Dannawi Z, Altaf F, Harshavardhana NS, El Sebaie H, Noordeen H. Early results of a remotely-operated magnetic growth rod in early-onset scoliosis. Bone Joint J 2013;95-B:75-80. [Google Scholar]
- 3.Hickey BA, Towriss C, Baxter G, Yasso S, James S, Jones A, et al. Early experience of MAGEC magnetic growing rods in the treatment of early onset scoliosis. Eur Spine J 2014;23 Suppl 1:S61-5. [Google Scholar]
- 4.Wu AM, Cheung JP, Cheung KM, Cheung KM, Lin JL, Jin HM, et al. Minimum 2-year experience with magnetically controlled growing rods for the treatment of early-onset scoliosis: A systematic review. Asian Spine J 2019;13:682-93. [Google Scholar]
- 5.Rushton PR, Siddique I, Crawford R, Birch N, Gibson MJ, Hutton MJ. Magnetically controlled growing rods in the treatment of early-onset scoliosis: A note of caution. Bone Joint J 2017;99-B:708-13. [Google Scholar]
- 6.Hirst A, Philippou Y, Blazeby J, Campbell B, Campbell M, Feinberg J, et al. No surgical innovation without evaluation: Evolution and further development of the IDEAL framework and recommendations. Ann Surg 2019;269:211-20. [Google Scholar]
- 7.Jones CS, Stokes OM, Patel SB, Clarke AJ, Hutton M. Actuator pin fracture in magnetically controlled growing rods: Two cases. Spine J 2016;16:e287-91. [Google Scholar]
- 8.Shaw KA, Hire JM, Kim S, Devito DP, Schmitz ML, Murphy JS. Magnetically controlled growing instrumentation for early onset scoliosis: Caution needed when interpreting the literature. World J Orthop 2019;10:394-403. [Google Scholar]
- 9.Rushton PR, Smith SL, Kandemir G, Forbes L, Fender D, Bowey AJ, et al. Spinal lengthening with magnetically controlled growing rods: data from the largest series of explanted devices. Spine (Phila Pa 1976) 2020;45:170-6. [Google Scholar]
- 10.Bess S, Akbarnia BA, Thompson GH, Sponseller PD, Shah SA, El Sebaie H, et al. Complications of growing-rod treatment for early-onset scoliosis: Analysis of one hundred and forty patients. J Bone Joint Surg Am 2010;92:2533-43. [Google Scholar]
- 11.Teoh KH, von Ruhland C, Evans SL, James SH, Jones A, Howes J, et al. Metallosis following implantation of magnetically controlled growing rods in the treatment of scoliosis: A case series. Bone Joint J 2016;98-B:1662-7. [Google Scholar]
- 12.Yilgor C, Efendiyev A, Akbiyik F, Demirkiran G, Senkoylu A, Alanay A, et al. Metal ion release during growth-friendly instrumentation for early-onset scoliosis: A preliminary study. Spine Deform 2018;6:48-53. [Google Scholar]
- 13.Thakar C, Kieser DC, Mardare M, Haleem S, Fairbank J, Nnadi C. Systematic review of the complications associated with magnetically controlled growing rods for the treatment of early onset scoliosis. Eur Spine J 2018;27:2062-71. [Google Scholar]
- 14.NuVasive Inc. Urgent Field Safety Notice/MAGEC System; 2019. Available from: https: //www.hpra.ie/docs/ default-source/ field-safety-notices /june-2019/ v40583_fsn.pdf?sfvrsn=2. [Google Scholar]
- 15.Medicines and Healthcare Products Regulatory Agency. Spinal Implant: MAGEC System Model X Rods Risk of Failure in Use (MDA/2020/010); 2020. Available from: https:// www.gov.uk/drug-device-alerts/ spinal-implant-magec- system-model- x-rods- risk-of- failure-in- use-mda- 2020-010#manufacturer -contacts. [Google Scholar]









