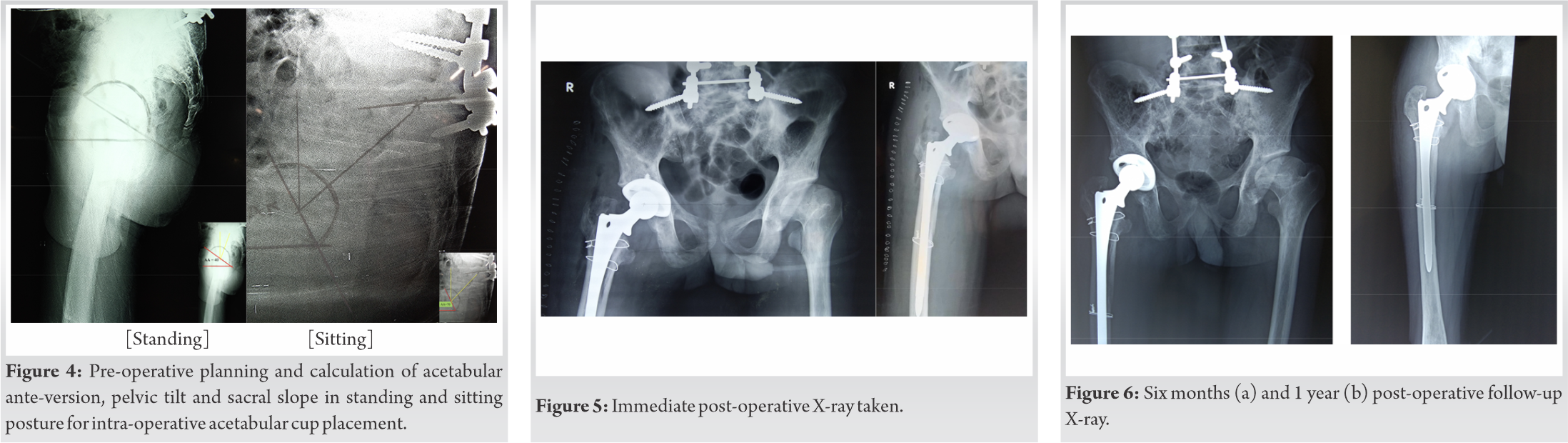
Well-constructed preoperative planning can help us tackle the anticipated intra-operative complications and post-operative rehabilitation in this unique patient group.
Dr. D J Vinay, Department of Orthopaedics, Sri Ramachandra Medical College and Research institute, Chennai, Tamil Nadu, India. E-mail: dr.vinaydj93@gmail.com
Introduction: Avascular necrosis of femoral head secondary to rare metabolic Gaucher’s disease (GD) can cause debilitating hip arthritis in young adults. It is an autosomal recessive disorder caused due to deficiency of lysosome enzyme glucocerebrosidase resulting in accumulation of its substrate in macrophages. The activated macrophages or the Gaucher cells causes hepatosplenomegaly, anemia, and thrombocytopenia. Extensive marrow involvement causes bony deformity, necrosis, and pathological fractures in non-neuropathic GD. Total hip replacement (THR) for young adult with secondary arthritis due to avascular necrosis (AVN) of femoral head in GD is complex and has high failure rate. As the abnormal cell infiltration involves both femoral head and the acetabulum. It becomes even more challenging, when associated spinopelvic fusion preexists. The altered biomechanics needs special attention to the anteversion of the cup placement and deciding the combined ante-version angle (CAVA).
Case Presentation:We report a case of GD with avascular necrosis of the femoral head, who underwent spinopelvic fusion to address his osteonecrosis of lumbar vertebra. Previously unreported, here we will discuss the pre-operative radiological evaluation and other intra-operative challenges in the management of GD post-enzyme replacement therapy (ERT) with secondary hip arthritis by THR.
Conclusion:Hip replacement surgery in patients with Gaucher disease related to secondary arthritis restores pain free mobility. Despite the young age of the patients with GD, prognosis remains better with THR after enzyme replacement therapy. The pre-operative planning, anticipation of complications in metabolically abnormal hip joints makes it a complex primary THR. However, in patients with the spinopelvic fusion placement of the cup, at the narrow range of angle of version with altered spinopelvic rhythm plays an important role in post-operative prosthetic hip stability and patient mobility.
Keywords:Gaucher’s disease, spinopelvic fusion, total hip arthroplasty, enzyme replacement therapy.
Avascular necrosis of femoral head is a feature of non- neuropathic type of Gaucher disease (GD) [1]. It is an autosomal recessive inherited lysosomal disorder, due to deficiency of lysosome enzyme glucocerebrosidase [2]. It results in accumulation of its substrate in macrophages that causes hepatosplenomegaly, anemia, thrombocytopenia, and multi organ dysfunction. The skeletal disorders in GD is due to bone marrow infiltration that affects the bone turn over resulting in multiple bone necrosis, deformities of long bone, and pathological fractures [3]. Mutations responsible for the enzymatic deficiency have been identified in the human glucocerebrosidase gene (chromosome 1q22). Literatures describe three different subtypes of GD [1, 4]. The most prevalent form is type 1 non-neuropathic type [4]. Infantile type 2 is the most lethal type with survival of 6 months to 1 year of life. Type 3 neuronal form has intermediate features of neuronal involvement. Even with type 1 spectrum of features may include late onset of neurological phenotype suggesting GD is a continuam of disease state [4]. Glucosylceramide accumulated macrophages transform them into ‘Gaucher cells’ when infiltrates the bone marrow, causes failure in remodeling formation of lytic bone cysts, subchondral bone cyst, and deformity of distal femur (Erlenmeyer flask deformity) Femoral head osteonecrosis is the most common symptomatic skeletal lesion [4, 5, 6] with the secondary arthritis of the hip causes disability in activity of daily life (ADL). Pathologic fractures, deformity of long bones, contracture, bone crisis, and marrow suppression are few other skeletal issues in GD [7]. It is common to notice vitamin D deficiency compromising the bone health [7, 8]. Enzyme replacement therapy (ERT) remains the specific treatment for GD. Non-operative pain management helps in the early stages of hip disease [7, 8, 9]. THR for severe hip arthritis secondary to osteonecrosis in GD becomes warranted at young age to improve ADL. Although cemented THR has been reported, post-enzyme replacement in GD uncemented THR has shown better results [10]. Prior spinopelvic fusion to address lower lumbar osteonecrosis increases the complexity of THR. Changes in spinal balance as a result of spinopelvic fusion can alter pelvic rotation through changes in pelvic tilt and obliquity [11]. During sitting and standing the resulting functional malposition of the acetabulam related to altered pelvic tilt and version can cause hip instability.
We report a case of 32-year-old male who presented with complaints of the right-sided hip pain for a period of 6 months. Insidious onset of dull and diffuse pain, gradually progressed that aggravated on weight bearing activities. The ability to carry out his daily activities deteriorated due to the stiffness and reduced mobility of his right hip. He had significant limp and needed a walker support even for his indoor mobility. There was no history of trauma, fever, night pain, or associated motor or sensory deficits. He was diagnosed to have Gaucher’s disease and has underwent splenectomy 10 years before this consultation. He underwent spinopelvic fusion between the levels L3-S1 for an L5 pathological fracture with osteonecrosis presented as vertebra plana 4 years prior to the hip problems (Fig. 1). He was on disease modifier trial drug Arimoclomal for a period of 1 year prior to his consultation.
On general examination, he was a thin built individual. Had healed scar over the lower back with prominence of screws on the left lumbar side with no scar tenderness on palpation. Local examination showed fixed flexion deformity of the right hip of 30o with loss of lumbar lordosis (Fig. 2a). Range of movements at his right hip was 30-70o and had gross painful restriction of rotational movements. Also he had painfully limited abduction and adduction movement with differential rotation. His spine examination revealed restricted lumbar flexion secondary to spinopelvic fusion but normal neurological status. On initial radiological examination, X-ray pelvis with bilateral hips and X-ray lumbo-sacral spine was taken. He had sclerotic femur head with reduced joint space suggestive of avascular necrosis (AVN) of the right femoral head with secondary osteoarthritis of the hip joint (Fig. 2b and 3). 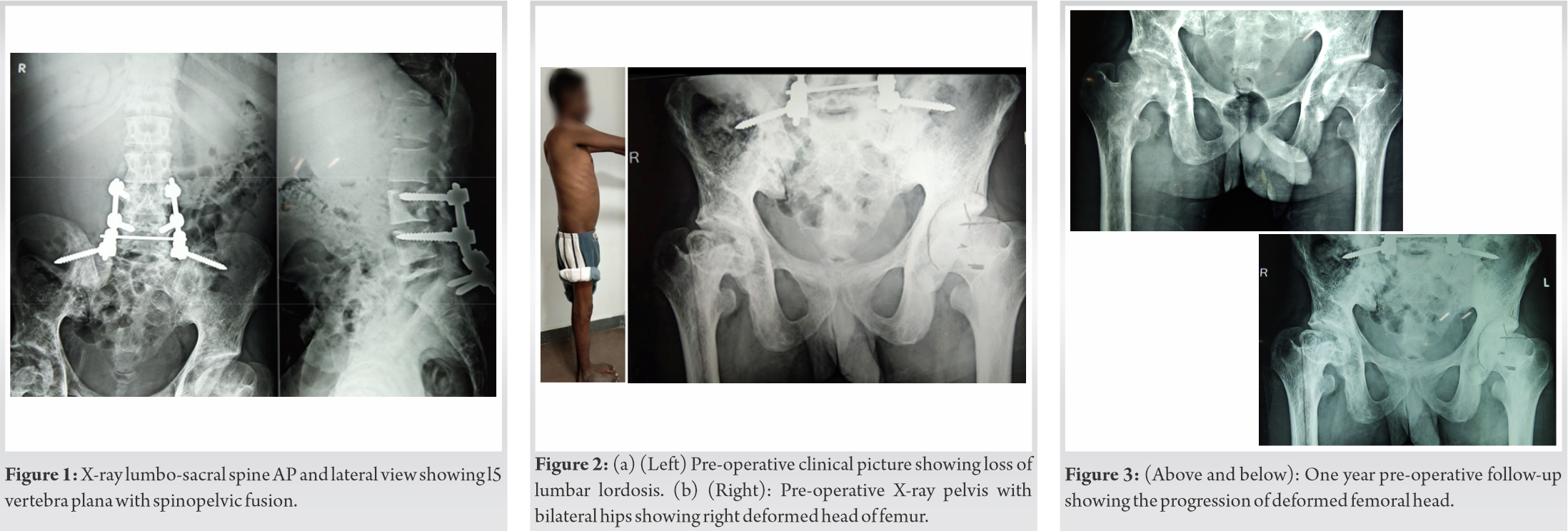
Gaucher’s disease is a rare genetic disorder with autosomal recessive inheritance pattern. The activated macrophages or the Gaucher cells causes hepatosplenomegaly, anemia, thrombocytopenia, and involvement of bone marrow and the skeletal system [1, 2]. The skeletal features are attributed to amalgamation of infiltration, edema, and ischemia. With the progression of disease, the infiltration extends from the axial skeleton to the extremities, involving more of lower limbs than the upper extremity [2, 3, 4].
Involvement of the femur, humerus, and vertebral bodies due to osteonecrosis induced collapse of the joint [3, 4, 5]. Proximal part of the bones is more commonly involved than the distal portion. Femoral head osteonecrosis is the most common symptomatic skeletal lesion presenting in young individuals that is significant enough in hampering their daily activities. Ischemia due to secondary chronic infarction is the possible mechanism, the progression of which cannot be stopped [6, 7].
Since degenerative hip changes are consistently noted in patients of Gaucher’s disease at a relatively young age, it becomes crucial in decision making if and when to undergo total hip arthroplasty. These patients have a vulnerable skeleton with a heterogenicity of pathological conditions including osteopenia and osteoporosis. Starting on enzyme replacement therapy at least 1–2 years before total hip replacement was noted to facilitate bone remodeling and allowed implantation of implant components in young patients [5, 6, 7, 8, 9]. The Gaucher cell infiltrate was noted to be reduced which enables us to prefer an uncemented total hip arthroplasty over cemented implant component for young adult THR [10]. Management of degenerative hip in patients with coexisting spine pathology involves a complex decision-making process and as a result comprehending the spinopelvic relationship is critical [11].
It is a complex relation between the sacral slope (SS), pelvic tilt (PT) and pelvic incidence (PI). The pelvic incidence is a constant parameter. The increased sacral slope during standing will be compensated with decreased pelvic tilt and while sitting decreased sacral slope will be compensated by increased pelvic tilt.
The normal mean standing to sitting change in PT is 20° and corresponding acetabular ante-version (AA) change by 15.6° is noted [11]. Greater than 30° difference in PT results in hyper-mobile joint with excess ante-version in flexion. Lesser than 10° difference in PT results in stiff spinopelvic rhythm, that is prone for anterior impingement and posterior dislocation [11]. In patients with spinopelvic fusion, the change in sacral slope is minimal. The compensatory increase in the pelvic tilt is noted between sitting and standing position and hence the acetabular ante-version (AA) will be dynamic from standing to sitting.
This compensatory increase in pelvic tilt with upright position essentially increases the range of AA and hence increase the risk of dislocation for a total hip arthroplasty [12]. The summative AA has to be retroverted by 1 degree for every 3 degree of lumbar lordosis or for every 1 degree of pelvic tilt correction. In our case the variation in AA from flexion to extension was maximum of 30 degree and hence preferable cup was placed in slight retroverted position to accommodate wide range of needed AA. The dislocation rate of a total hip arthroplasty in a fused lumbosacral patient is around 3% in the first year and 7.5% at 2 years [13, 14, 15]. Planning to accommodate the variable acetabular ante-version will minimize the risk ratio in such cases with altered spinopelvic fusion.
Overall, hip replacement surgery in patients with Gaucher disease has a good prognosis despite the younger age of patients and their complicated medical background. Hence, keeping these factors in mind, pre-operative planning and the timing of surgery plays an important aspect. Enzyme replacement therapy prior the surgical procedure enables us to opt for uncemented implant fixation. Our patient in the study had a spinopelvic fusion done and the pre-operative planning of the approach and the placement of the cup at the narrow range of angle was not to be erred. A detailed preoperative workup with the necessity implant instrumentation is needed for management of this unique patient group, taking into account the variables that contribute to increased dislocation, as well as the application of clinical judgment in scheduling and executing the surgery.
A detailed pre-operative workup with the necessity implant instrumentation is needed for management of this unique patient group, taking into consideration the factors that are involved in greater dislocation, and the use of clinical judgment in timing and performing the surgery.
References
- 1.Charrow J, Andersson HC, Kaplan P, Kolodny EH, Mistry P, Pastores G, et al. Enzyme replacement therapy and monitoring for children with type I Gaucher disease: Consensus recommendations. J Pediatr 2004;144:112-20. [Google Scholar]
- 2.Hughes D, Mikosch P, Belmatoug N, Carubbi F, Cox T, Goker-Alpan O, et al. Gaucher disease in bone: From pathophysiology to practice. J Bone Miner Res 2019;34:996-1013. [Google Scholar]
- 3.Stowens DW, Teitelbaum SL, Kahn AJ, Barranger JA. Skeletal complications of Gaucher disease. Medicine (Baltimore) 1985;64:310-22. [Google Scholar]
- 4.Lineri S, Castaman G. Clinical manifestation and management of Gauchers disease. Clin Cases Min Bone Metab 2015;12:157-64. [Google Scholar]
- 5.Bubbar V, Heras FL, Amato D, Pritzker KP, Gross AE. Total hip replacement in Gaucher’s disease: Effects of enzyme replacement therapy. J Bone Joint Surg Br 2009;91:1623-7. [Google Scholar]
- 6.Itzchaki M, Lebel E, Dweck A, Patlas M, Hadas-Halpern I, Zimran A, et al. Orthopedic considerations in Gaucher disease since the advent of enzyme replacement therapy. Acta Orthop Scand 2004;75:641-53. [Google Scholar]
- 7.Pastores GM, Inhorn TA. Skeletal complications of Gaucher disease: Pathophysiology, evaluation and treatment. Semin Hematol 1995;32:20-7. [Google Scholar]
- 8.Mikosch P, Reed M, Stettner H, Baker R, Mehta AB, Hughes DA. Patients with Gaucher disease living in England show a high prevalence of vitamin D insufficiency with correlation to osteodensitometry. Mol Genet Metab 2009;96:113-20. [Google Scholar]
- 9.Mankin HJ, Doppelt SH, Rosemberg AE, Barranger JA. Metabolic bone disease in patients with Gaucher disease. In: Avioli LV, Krane SM, editors. Metabolic Bone Disease and Clinically Related disorders. 2nd ed. Philadelphia, PA: WB Saunders Company; 1990. p. 730-52. [Google Scholar]
- 10.Cohen D, Kogan D, Rubin A, Zimran A, Lebel E. Longevity of total hip arthroplasty implants in patients with Gaucher disease. HIP Int 2020;30:147-51. [Google Scholar]
- 11.Lee SH, Lim CW, Choi KY, Jo S. Effect of spine-pelvis relationship in total hip arthroplasty. Hip Pelvis 2019;31:4-10. [Google Scholar]
- 12.Gausden EB, Parhar HS, Popper JE, Sculco PK, Rush BN. Risk factors for early dislocation following primary elective total hip arthroplasty. J Arthroplasty 2018;33:1567-71.e2. [Google Scholar]
- 13.Sultan AA, Khlopas A, Piuzzi NS, Chughtai M, Sodhi N, Mont MA. The impact of spino-pelvic alignment on total hip arthroplasty outcomes: A critical analysis of current evidence. J Arthroplasty 2018;33:1606-16. [Google Scholar]
- 14.Marcucci G, Zimran A, Bembi B, Kanis J, Reginster JY, Rizzoli R, et al. Gaucher disease and bone manifestations. Calcif Tissue Int 2014;95:477-94. [Google Scholar]
- 15.Lazennec JY, Charlot N, Gorin M, Roger B, Arafati N, Bissery A, et al. Hip-spine relationship: A radio-anatomical study for optimization in acetabular cup positioning. Surg Radiol Anat 2004;26:136.e44. [Google Scholar]