A systematic line of investigation followed by tumor clearance with adequate margin will help in recurrence-free management of rare tumors such as haemangioendothelioma.
Dr. Girinivasan Chellamuthu, Research Associate, Orthopaedic Research Group, Church Road, Ramanathapuram, Coimbatore - 641 045, Tamil Nadu, India. E-mail: giri.c.nivasan@gmail.com
Introduction: Lytic lesions of the proximal tibia include a plethora of differential diagnoses. The most common ones are the Giant cell tumor, fibrous dysplasia, adamantinoma, chondromyxoid fibroma, and osteoblastoma. The rarer ones include vascular tumors such as hemangioma and hemangioendothelioma. A systematic line of investigations is essential to pick up the right diagnosis especially in case of rarer conditions. In this background, we present a case of lytic lesion of the proximal tibia which turned out to be epitheloid hemangioendothelioma (EHE).
Case Report: A 37-year-old female presented with pain and swelling in the left knee for 2 years. On examination, the patient had a 3 × 4 cm firm, non-tender, and well-defined swelling on the anterolateral aspect of the proximal tibia. X-ray showed a lytic lesion of the proximal tibia. Magnetic resonance imaging was suggestive of a giant cell tumor. However, the biopsy revealed a rare diagnosis of epithelioid hemangioendothelioma.
Conclusion: The patient was managed with wide excision. In a young adult, arthrodesis may not be the best option; hence, we reconstructed the joint with a custom mega prosthesis (CMP). At 3 years follow-up, our patient had no signs of recurrence. To our best knowledge, this is the first report on the use of CMP in a case of EHE
Keywords: Hemangioendothelioma, lytic lesion, CMP
Lytic lesions of the proximal tibia include a plethora of differential diagnoses [1]. The most common one in a young adult is the Giant cell tumor. The other conditions are fibrous dysplasia, adamantinoma, chondromyxoid fibroma, and osteoblastoma [1, 2]. The rarer ones include vascular tumors such as hemangioma and hemangioendothelioma [3]. Most of these tumors are benign. Some are locally aggressive while a few can cause distant metastasis [1, 3]. Thus, the management of each condition and its prognosis differs. A systematic line of investigations is essential to pick up the right diagnosis especially in case of rarer conditions. In this background, we present a case of lytic lesion of the proximal tibia which turned out to be epitheloid hemangioendothelioma (EHE). The EHE is an extremely rare tumor accounting for 1% of all vascular tumors with an estimated prevalence of 1 in 1 million [4, 5]. It is locally aggressive, characterized by the formation of atypical endothelial cells, and has a low to intermediate grade malignant potential [4]. Wide excision of tumor and reconstruction with custom mega prosthesis (CMP) was done.
A 37-year-old female presented with pain and swelling in the left knee for 2 years. The patient experienced an increased intensity of pain and noticed an increase in the size of the tumor for the past 3 months.On examination, the patient had a 3 × 4 cm firm, non-tender, and well-defined swelling on the anterolateral aspect of the proximal tibia. Her knee movements were full and were associated with pain in the terminal range of flexion. She had no distal neurovascular deficit.
Investigations
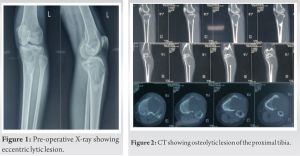
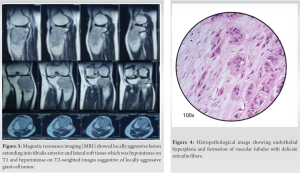
The knee radiograph and computer tomography (CT) showed an eccentric lytic epihyseo-metaphyseal lesion with well-defined margins confined to the proximal tibia (Fig. 1, 2). Magnetic resonance imaging (MRI) showed locally aggressive lesion extending into tibialis anterior, lateral soft tissue, and fibular head which was hypointense on T1 and hyperintense on T2-weighted images suggestive of locally aggressive giant cell tumor (Fig. 3). Tru-cut biopsy showed an infiltrative tumor with nests of the spindle to epithelioid cells in a myxoid stroma. Some cells showed prominent cytoplasmic vacuoles. There was a low mitotic activity with mild pleomorphism. The cells were positive for CD31 and FLI1. Whole-body CT was done to exclude multifocal lesions and metastasis. A diagnosis of unifocal epithelioid hemangioendothelioma was made.
Treatment
The case was discussed in the Institute’s Tumor Board. A decision of wide local excision and reconstruction of the joint with CMP was made. Using the pre-operative radiographs CT and MRI, measurement of tumor size was made and a CMP was ordered accordingly.
Operative procedure
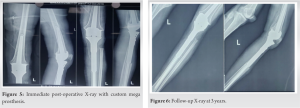
Under spinal anesthesia, with the patient in the supine position, parts were prepped and draped. Through an incision including the tru-cut biopsy site, the tumor was exposed. The margins of the tumor were carefully dissected out. About 4 cm below the bony margin, the proximal tibia cut was made. A fibular cut is made below the fibular head. The tumor along with a cuff of normal soft tissue was removed. Histopathological examination demonstrated endothelial hyperplasia and formation of vascular tubules with delicate reticulin fibers (Fig 4). The wound was irrigated with hydrogen peroxide. Proximally the incision was extended to expose the knee joint. An appropriate distal femur cut was made and the CMP was applied (Fig. 5).
Ankle pump exercises were started immediately. Continuous passive mobilization and knee bending exercises were advised after suture removal. Weight-bearing walking was advised after 4 weeks.
Outcome and follow up
The patient had no neurovascular deficit. At 3-years follow-up, there is no sign of recurrence (Fig. 6). The range of knee movements is 0 to 90°. There is no sign of loosening of the prosthesis.
EHE is a rare tumor of vascular endothelium classified by the World Health Organization as a low-grade malignant vascular sarcoma [6]. The tumor was first differentiated from angiosarcoma and described by Thomas in 1942 [7]. Its etiology is not clear. However, it is characterized by a translocation between chromosomes 1 and 3 resulting in a fusion protein containing transcription regulator 1 and the calmodulin-binding transcription activator 1, leading to constitutive activation and malignant transformation [5, 8]. It can occur in any age group between 10 and 60 years. The median age is 36 years [3, 9]. It can be unifocal or multifocal. About 50% of the tumor is multifocal [10]. There is a strong male preponderance with a male to female ratio of 4:1 [5]. The lesion can by its nature occur anywhere in the body. The most common form of diagnosis (70%) is incidental as a pulmonary nodule in a chest radiograph. Isolated bone involvement as in our case is seen in 14% of cases [9]. The most common site of involvement is the lower limb. Cases have also been reported in the spine, ribs, and upper limbs [2, 11, 12]. Clinically, the patient can present with bone pain, swelling, pathological fracture, or due to systemic involvement with liver disease or symptoms such as dyspnea. The patient may have bone marrow suppression, weight loss, or neurological manifestations in case of spine involvement [9, 13, 14, 15, 16, 17, 18, 19].
Like all vascular tumors, it appears as a lytic lesion in X-rays. There are no specific features in MRI to look for. The lesion is low to intermediate intensity on T1 and is hyperintense on T2-weighted images. There is a homogeneous enhancement in the presence of gadolinium-based contrast material. The serpentine pattern indicating vascular structures is not typically seen [17]. The differential diagnosis includes Giant cell tumors, angiomatosis, langerhans cell histiocytosis, angiosarcoma, infection, myeloma, metastasis, and lymphoma [9, 17]. The lesion (Fig. 3) is described to have classically three distinct features [20, 21].
1. Endothelial hyperplasia
2. Formation of vascular tubules with delicate reticulin fibers
3. Blister cells-cells with distinct vacuolated cytoplasm
Immunohistochemically, the cells are positive for several markers. They include CD31 (100%) CD34 (85%), FLI1 (100%), ERG (98%), and Claudin-1 (88%) among others [22, 23, 24]. Most lesions are indolent. However, 20%–30% of the tumors metastasize and mortality is approximately 15%. The most common site of metastasis is the lungs [25].
Given the rarity of the disease, large studies evaluating treatment strategies are not available [9]. In isolated lesions, surgical resection is the best option. In multifocal asymptomatic lesions, even spontaneous resolution has been reported [26]. In symptomatic multifocal lesions, a multimodality approach with chemotherapy, immunotherapy, targeted therapy, and radiotherapy should be followed [9, 12]. Various chemotherapeutic agents described as useful in case reports include carboplatin, etoposide, pemetrexed, and bevacizumab [27, 28, 29]. Radiotherapy should be avoided whenever possible as radiation-induced sarcomatous changes have been reported [12]. Available literature suggests wide excision as described by Enneking as the best possible surgical treatment [12, 30]. The recurrence rate after intralesional curettage has been much higher when compared to wide excision [12]. However, the reconstruction options have been not widely discussed. To our best knowledge, this is the first report on the use of CMP in a case of EHE. Since the location of the tumor was epiphyseo-metaphyseal, salvage of the joint was not possible. In a young adult, arthrodesis may not be the best option; hence, we reconstructed the joint with a CMP. At 3-year, follow-up our patient had no signs of recurrence.
We have presented the successful management of an extremely rare case. The key for diagnosis was the systematic evaluation of the lesion. Tumor clearance with adequate margin followed by the application of CMP helped us achieve recurrence-free knee with good function. In treating locally aggressive tumors, the treating setup must be equipped with such arthroplasty options.
EHE is an extremely rare tumor. A systematic approach of investigations is required for the diagnosis of such a rare tumor. Wide excision is the surgery of choice in unifocal lesions. The multimodality approach should be used in multifocal lesions. In periarticular lesions, in young adults, CMP can be used for reconstruction.
References
- 1.Salzer-Kuntschik M. Differential diagnosis of giant cell tumor of bone. Verh Dtsch Ges Pathol 1998;82:154-9. [Google Scholar]
- 2.Neto FA, Teixeira MJ, Araújo LH, Ponte CE. Knee bone tumors: Findings on conventional radiology. Radiol Bras 2016;49:182-9. [Google Scholar]
- 3.van IJzendoorn DG, Bovée JV. Vascular tumors of bone: The evolvement of a classification based on molecular developments. Surg Pathol Clin 2017;10:621-35. [Google Scholar]
- 4.Gherman CD, Fodor D. Epithelioid hemangioendothelioma of the forearm with radius involvement. Case report. Diagn Pathol 2011;6:120. [Google Scholar]
- 5.Sardaro A, Bardoscia L, Petruzzelli MF, Portaluri M. Epithelioid hemangioendothelioma: An overview and update on a rare vascular tumor. Oncol Rev 2014;8:259. [Google Scholar]
- 6.Fletcher CD, Bridge JA, Hogendoorn P, Mertens F. Vascular Tumors. Lyon (France): International Agency for Research on Cancer; 2013. [Google Scholar]
- 7.Thomas A. Vascular tumors of bone: Pathological and clinical study of 27 cases. Surg Gynec Obstet 1942;74:777-95. [Google Scholar]
- 8.Tanas MR, Sboner A, Oliveira AM, Erickson-Johnson MR, Hespelt J, Hanwright PJ, et al. Identification of a disease-defining gene fusion in epithelioid hemangioendothelioma. Sci Transl Med 2011;3:98ra82. [Google Scholar]
- 9.Rosenberg A, Agulnik M. Epithelioid hemangioendothelioma: Update on diagnosis and treatment. Curr Treat Options Oncol 2018;19:19. [Google Scholar]
- 10.Campanacci M, Boriani S, Giunti A. Hemangioendothelioma of bone: A study of 29 cases. Cancer 1980;46:804-14. [Google Scholar]
- 11.Luzzati A, Gagliano F, Perrucchini G, Scotto G, Zoccali C. Epithelioid hemangioendothelioma of the spine: Results at 7 years of average follow-up in a series of 10 cases surgically treated and a review of the literature. Eur Spine J 2015;24:2156-64. [Google Scholar]
- 12.Angelini A, Mavrogenis AF, Gambarotti M, Merlino B, Picci P, Ruggieri P. Surgical treatment and results of 62 patients with epithelioid hemangioendothelioma of bone. J Surg Oncol 2014;109:791-7. [Google Scholar]
- 13.Asirvatham JR, Brody J, Wang X, Chandok A, Zhang X. Epithelioid hemangioendothelioma involving bone marrow presenting with persistent anemia. Am J Hematol 2013;88:530. [Google Scholar]
- 14.Lau K, Massad M, Pollak C, Rubin C, Yeh J, Wang J, et al. Clinical patterns and outcome in epithelioid hemangioendothelioma with or without pulmonary involvement: Insights from an internet registry in the study of rare cancer. Chest 2011;140:1312-8. [Google Scholar]
- 15.Bagan P, Hassan M, Le Pimpec BF, Peyrard S, Souilamas R, Danel C, et al. Prognostic factors and surgical indications of pulmonary epithelioid hemangioendothelioma: A review of the literature. Ann Thorac Surg 2006;82:2010-3. [Google Scholar]
- 16.Gómez-Arellano LI, Ferrari-Carballo T, DomínguezMalagón HR. Multicentric epithelioid hemangioendothelioma of bone. Report of a case with radiologic-pathologic correlation. Ann Diagn Pathol 2012;16:43-7. [Google Scholar]
- 17.Larochelle O, Perigny M, Lagace R, Dion N, Giguere C. Best cases from the AFIP: Epithelioid hemangioendothelioma of bone. Radiographics 2006;26:265-70. [Google Scholar]
- 18.Albakr A, Schell M, Drew B, Cenic A. Epithelioid hemangioendothelioma of the spine: Case report and review of the literature. J Spine Surg 2017;3:250-9. [Google Scholar]
- 19.Ma J, Wang L, Mo W, Yang X, Xiao J. Epithelioid hemangioendothelioma of the spine: Clinical characters with middle and long-term follow-up under surgical treatments. Eur Spine J 2011;20:1371-6. [Google Scholar]
- 20.Stout AP. Hemangio-endothelioma: A tumor of blood vessels featuring vascular endothelial cells. Ann Surg 1943;118:445-64. [Google Scholar]
- 21.Weiss SW, Enzinger FM. Epithelioid hemangioendothelioma: A vascular tumor often mistaken for a carcinoma. Cancer 1982;50:970-81. [Google Scholar]
- 22.Miettinen M, Wang ZF, Paetau A, Tan SH, Dobi A, Srivastava S, et al. ERG transcription factor as an immunohistochemical marker for vascular endothelial tumors and prostatic carcinoma. Am J Surg Pathol 2011;35:432-41. [Google Scholar]
- 23.Gill R, O’Donnell RJ, Horvai A. Utility of immunohistochemistry for endothelial markers in distinguishing epithelioid emangioendothelioma from carcinoma metastatic to bone. Arch Pathol Lab Med 2009;133:967-72. [Google Scholar]
- 24.Miettinen M, Rikala MS, Rys J, Lasota J, Wang ZF. Vascular endothelial growth factor receptor 2 as a marker for malignant vascular tumors and mesothelioma: An immunohistochemical study of 262 vascular endothelial and 1640 nonvascular tumors. Am J Surg Pathol 2012;36:629-39. [Google Scholar]
- 25.Deyrup AT, Tighiouart M, Montag AG, Weiss SW. Epithelioid hemangioendothelioma of soft tissue: A proposal for risk stratification based on 49 cases. Am J Surg Pathol 2008;32:924-7. [Google Scholar]
- 26.Kitaichi M, Nagai S, Nishimura K, Itoh H, Asamoto H, Izumi T, et al. Pulmonary epithelioid haemangioendothelioma in 21 patients, including three with partial spontaneous regression. Eur Respir J 1998;12:89-96. [Google Scholar]
- 27.Kanemura S, Kuribayashi K, Moriya Y, Shimizu S, Tsujimura T, Nakano T. Pemetrexed for epithelioid haemangioendothelioma of the pleura. Respirol Case Rep 2016;4:e00191. [Google Scholar]
- 28.Pinet C, Magnan A, Garbe L, Payan MJ, Vervloet D. Aggressive form of pleural epithelioid haemangioendothelioma: Complete response after chemotherapy. Eur Respir J 1999;14:237. [Google Scholar]
- 29.Merikas E, Grapsa D, Dikoudi E, Gkiozos I, Boura P, Charpidou A, et al. Epithelioid hemangioendothelioma treated with bevacizumab: A case series. Cancer Treat Commun 2015;4(Supplement C):59-64. [Google Scholar]
- 30.Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res 1986;204:9-24. [Google Scholar]