Patients with multiple pathologic or refractory fractures, even if presenting after trauma should be carefully assessed for lesser-known underlying tumor-induced causes of osteomalacia such as a phosphaturic mesenchymal tumor.
Abrianna S Robles, Department of Orthopaedic Surgery, Cedars Sinai Medical Center, Los Angeles, California. E-mail: abrianna.robles@gmail.com
Introduction: Phosphaturic mesenchymal tumor (PMT) is a rare benign tumor (500 cases to date) that can present in combination with a paraneoplastic syndrome called tumor-induced osteomalacia (TIO). To the best of our knowledge, it is the first case to date that presented as an orthopedic trauma patient.
Case Report: This is a case of a 61-year-old male who initially presented as a polytrauma patient, but further investigation revealed a PMT causing TIO. This report describes his initial diagnosis and management from 2015 to 2021.
Conclusion: TIO resultant of PMT may lead to severe bone pain, impending fractures, and delayed or misdiagnosis. This case demonstrates the importance of careful diagnosis and a team-based approach to managing PMT and its sequelae.
Keywords: Pathological fracture, phosphaturic mesenchymal tumor, tumor-induced osteomalacia
Phosphaturic mesenchymal tumor (PMT) is a rare benign tumor (500 cases to date) that can present in combination with a paraneoplastic syndrome called tumor-induced osteomalacia (TIO) [1, 2, 3, 4, 5, 6, 7, 8]. TIO causes fatigue, bone pain, weakness, or impending fractures secondary to severe hypophosphatemia and osteomalacia [5, 9]. Tumor cells stimulate upregulation of fibroblast growth factor 23 (FGF-23), preventing formation of 1, 25-dihydroxyvitamin D3 (calcitriol), which inhibits reabsorption of phosphate in the kidneys [5, 7, 9, 10]. PMTs have been reported in bone and soft tissue, including the thigh, foot, hip, head, neck, and spine [1, 2, 3, 4, 5, 9, 10, 11, 12, 13, 14, 15, 16, 17]. TIO may present with hypophosphatemia, elevated alkaline phosphatase (ALP), normal parathyroid hormone levels (PTH), and low-to-normal serum calcium levels [7, 18]. PMT should be suspected whenever non-familial hypophosphatemic osteomalacia is diagnosed [7, 19]. If there is clinical suspicion for PMT/TIO, physicians should conduct full radiographic and biochemical assessment. Due to the rarity of disease, most patients undergo years of misdiagnosis before appropriate treatment [7, 19, 20, 21]. Untreated, PMTs can progress to malignant tumors such as high-grade sarcomas [22]. This case examines a 61-year-old male presenting with a TIO-induced pathologic subtrochanteric femur fracture, contralateral subtrochanteric femur fracture, and impending humeral shaft fracture after 2 years of debilitating pain secondary to PMT. The patient provided consent for the publication of his case.
Initial presentation and work-up
A 61-year-old male with a medical history of diabetes mellitus Type II, diabetic neuropathy, and hypertension presented to the emergency department with bilateral lower extremity and right upper extremity pain after a mechanical fall resulting in the right lower extremity deformity and inability to ambulate. He reported 2 years of progressive pain and weakness in the bilateral thighs necessitating walker use, and 1 year of intermittent night sweats and bilateral shoulder pain (right greater than left). He denied weight loss, change in appetite, bisphosphonate use, osteoporosis, or previous cancers. Radiographs demonstrated a displaced transverse right subtrochanteric femur fracture at a stress fracture-like lesion, left incomplete transverse subtrochanteric fracture, and a large lytic lesion involving the right humeral shaft (Fig. 1). The patient’s history and multi-focal pathology were concerning for underlying malignancy. The course proceeded with biopsy and operative stabilization of his right femur. Definitive fixation of the left femur and right humerus was delayed until biopsy results and oncologic evaluation were obtained.
Initial surgical management
A lateral approach to the right femur was utilized and a superficial vastus lateralis muscle specimen and a bone specimen from the fracture site and surrounding soft tissues were collected and sent for frozen section. These were reported as benign hematoma and cancellous bone, so additional biopsy of the intramedullary canal was performed with the Reamer/Irrigator/Aspirator [RIA]© (Depuy Synthes, West Chester, PA) before nailing and fixation with a long trochanteric-entry cephalomedullary nail.
Oncologic evaluation
After initial procedure, the orthopedic oncology and hematology/oncology teams were consulted. Final pathology demonstrated simple marrow elements without evidence of malignancy. Given the lytic lesion in the right humerus, work-up included prostate specific antigen, serum/urine protein electrophoresis, parathyroid hormone-related protein, quantitative immunoglobulins, kappa/lambda light chains, carcinoembryonic antigen, and carbohydrate antigen 19–9. All markers were negative, but metabolic panel was notable for slightly low serum calcium at 7.7 mg/dL, elevated intact PTH at 114.2 PG/ML, and low phosphorus level at 1.1 mg/dl, suggesting paraneoplastic syndrome versus metabolic tumor (i.e., brown tumor of the parathyroid). The otolaryngology service was consulted and CT imaging demonstrated multiple rib and transverse process fractures but no primary tumor or lymphadenopathy. Positron emission tomography (PET) scan showed mild diffuse nonspecific metabolic activity. CT-guided biopsy of the 3.8 × 1.5 × 0.9 cm right humerus periosteal lesion revealed bland spindle cell proliferation suggesting mixed connective tissue PMT (Image 4). FGF-23 was not available at the institution, but somatostatin receptor 2A showed variable positivity supporting the diagnosis of PMT.
Subsequent surgical management
Following biopsy, the team proceeded with definitive management of the left subtrochanteric femur fracture and open biopsy and curettage of the right humeral lesion on hospital day 14. The patient underwent placement of a long trochanteric-entry cephalomedullary nail for the incomplete left subtrochanteric femur fracture with opening reamer biopsy. The humerus lesion was then localized under fluoroscopic guidance and an anterolateral approach was used. Following dissection to the brachialis, the muscle was incised at the defect and multiple specimens were obtained. Frozen section pathology revealed spindle cell process with few atypical cells (Fig. 2), but definitive diagnosis could not be made. During attempted curettage, the lesion was noted to be in contact with the radial nerve. Careful dissection from the nerve was performed and curettage and saucerization of the bone defect followed. The specimen was sent to pathology for definitive diagnosis and prophylactic nailing was deferred. Final diagnosis of benign mixed connective tissue PMT was made on hospital day 15 and prophylactic intramedullary nailing of the right humerus was performed on hospital day 16.
Remaining hospital course
Endocrinology was consulted following definitive surgical intervention. Phosphorous and Vitamin D supplementation were initiated, and an octreotide scan was performed to rule out additional foci of PMT. Quarterly endocrinology follow-up was scheduled. Medical and radiation oncology were consulted during hospitalization; however, no additional treatment was recommended before discharge to skilled nursing facility on hospital day 19.
Follow-up course
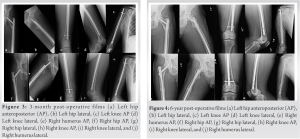
At the 3-, 6-, and 8-week post-operative appointments, the patient returned to the orthopedic trauma and oncology clinics noting improvement in pain and mobility. Radiographs were performed at orthopedic follow-up at 2–3-month intervals (Fig. 3). Endocrinology continued to manage his persistent osteomalacia. Despite medical management, he developed delayed union of the bilateral femur fractures and multiple nonoperatively treated osteomalacia-induced fractures (fifth metatarsal fracture and bilateral ilium fractures). Due to insurance, the patient was unable to continue endocrinologic follow-up at 15 months postoperatively and discontinued vitamin supplementation. Eighteen months following initial presentation, he returned to orthopedic oncology with increased pain and swelling in the right thigh and humerus concerning for tumor recurrence. PET scan revealed increased metabolic activity in the right humerus. Given previous excision, the patient underwent radiation (4500 cGy) to the right humeral lesion with resolution of pain without further lucency or fracture. Despite radiotherapy and vitamin supplementation, the patient continued to report right thigh pain 2-year post-surgery. Laboratory evaluation revealed PTH of 118.4 pg/mL and calcium of 8.1 mg/dL. His failure to return to normal PTH (despite imaging indicating resolution of the PMT) was concerning for alternative cause of hyperparathyroidism. Further work-up and bone stimulator were initiated. Endocrinologic and otolaryngologic evaluations for primary hyperparathyroidism were negative. Vitamin D, phosphate, and alendronate therapies were initiated to improve bone density. Within 5 months, his PTH decreased to 97.5 pg/mL, calcium increased to 9.7 mg/dL, and his femur fracture had healed with complete resolution of pain. The patient continues to follow-up with the orthopedic oncology and orthopedic trauma service now 6-year post-surgical intervention without recurrence (Fig. 4).
PMT/TIO can present with progressive myalgias, fatigue, and diffuse bone pain. It may lead to recurrent fractures [3, 4, 5, 6, 7, 8, 13]. In addition, various presenting locations and symptoms make diagnosis difficult as it may require full body imaging [7, 8, 23, 24, 25]. Zuo et al. reported that 11 of their 12 PMT patients were misdiagnosed before undergoing appropriate management. Remission of PMT has only been achieved with complete tumor resection, while recurrence has been successfully treated with resections, radiation, or medical therapy [6, 8, 23, 24, 25, 26]. This patient reported a 2-year history of debilitation with progression to walker use before low-energy pathologic femur fracture, incomplete contralateral femur fracture, and impending humerus fracture. The length-to-diagnosis of this patient is consistent with the literature, which reports 2.5 years to >20 years in some cases [10, 23, 27]. The patient’s prolonged symptoms were inaccurately attributed to diabetic neuropathy. This misdiagnosis contributed to the complex progression of his disease. On admission, the patient had labs concerning for normocalcemic hyperparathyroidism leading to osteomalacia. TIO is often misdiagnosed as primary hyperparathyroidism, leading to overlooking the culprit tumors [10]. This patient’s right humeral lesion allowed for more accurate assessment. Most cases of patients with PMT-induced TIO present with non-traumatic fractures [3, 11, 18, 24, 25]. This case is unique in that the patient initially presented as an orthopedic trauma patient with multiple affected extremities after a ground-level fall. Symptomatic treatment of TIO includes Vitamin D and phosphorus supplementation. Surgical resection is the gold standard for definitive treatment [5, 6, 7, 8]. >90% of cases treated with resection report complete recovery [1, 3, 6, 7, 17, 23, 24, 25, 26, 27, 28, 29, 30, 31]. Treatment may require adjuvant therapy including radiation [6, 10], monoclonal antibodies [27, 28], and medical therapy [6, 29, 30, 31]. Preoperatively, patients may be treated with octreotide to eliminate renal phosphate wasting [30]. Recurrence or persistent symptoms have been treated with oral phosphate, calcium, and calcitriol with varying results, and the use of anti-FGF23 monoclonal antibody KRN23 for treatment of unresectable TIO has been investigated [28]. This patient’s initial diagnosis and surgical management were timely and successful, but his outcome was dependent on resolution of insurance issues, radiotherapy, and re-establishment of endocrinologic care. This resulted in alendronate use, improved bone density, resolution of his tertiary hyperparathyroidism, and fracture healing.
PMT/TIO should be suspected in patients with multiple pathologic/refractory fractures and a biochemical profile of low serum phosphate, elevated ALP, normal PTH, and low-to-normal serum calcium levels. Heightened awareness of PMT/TIO can lead to more timely and accurate diagnoses, proper treatment, and improvement in quality of patient care and outcomes.
Patients with multiple pathologic or refractory fractures should be carefully assessed, as there may be a lesser-known underlying tumor-induced cause of osteomalacia such as a PMT. Providers should obtain a thorough orthopedic history and have a high degree of suspicion for these tumors leading to adequate laboratory and imaging workup to achieve a timely and accurate diagnosis.
References
- 1.Agarwal N, Kale SS, Kumari K. Tumor-induced osteomalacia due to a phosphaturic mesenchymal tumor in the cervical spine: A case report and literature review. Neurology India 2019;67:1334-40. [Google Scholar]
- 2.Arai R, Onodera T, Terkawi MA, Mitsuhashi T, Kondo E, Iwasaki N. A rare case of multiple phosphaturic mesenchymal tumors along a tendon sheath inducing osteomalacia. BMC Musculoskelet Disord 2017;18:79. [Google Scholar]
- 3.Aziz KT, McCarthy EF, Morris CD. Oncogenic osteomalacia secondary to a metastatic phosphaturic mesenchymal tumor in the talus: A case report and review of the literature. JBJS Case Connect 2017;7:e40. [Google Scholar]
- 4.De Beur SM. Tumor-induced osteomalacia. JAMA 2005;294:1260-7. [Google Scholar]
- 5.Carpenter TO. Oncogenic osteomalacia-A complex dance of factors. New Engl J Med 2003;348:1705-8. [Google Scholar]
- 6.Chong WH, Molinolo AA, Chen CC, Collins MT. Tumor-induced osteomalacia. Endocr Relat Cancer 2011;18:R53-77. [Google Scholar]
- 7.Deep NL, Cain RB, McCullough AE, Hoxworth JM, Lal D. Sinonasal phosphaturic mesenchymal tumor: Case report and systematic review. Allergy Rhinol (Providence) 2014;5:162-7. [Google Scholar]
- 8.Weidner N, Cruz DS. Phosphaturic mesenchymal tumors. A polymorphous group causing osteomalacia or rickets. Cancer 1987;59:1442-54. [Google Scholar]
- 9.Dezfulian M, Wohlgenannt O. Revision hip arthroplasty following recurrence of a phosphaturic mesenchymal tumor. J Surg Case Rep 2013;2013:rjt059. [Google Scholar]
- 10.Dutta D, Pandey RK, Gogoi R, Solanki N, Madan R, Mondal A, et al. Occult phosphaturic mesenchymal tumour of femur cortex causing oncogenic osteomalacia diagnostic challenges and clinical outcomes. Endokrynol Pol 2018;69:205-10. [Google Scholar]
- 11.Gandhi GY, Shah AA, Wu KJ, Gupta V, Shoraka AR. Tumor-induced osteomalacia caused by primary fibroblast growth factor 23 secreting neoplasm in axial skeleton: A case report. Case Rep Endocrinol 2012;2012:185454. [Google Scholar]
- 12.Honda R, Kawabata Y, Ito S, Kikuchi F. Phosphaturic mesenchymal tumor, mixed connective tissue type, non-phosphaturic variant: Report of a case and review of 32 cases from the Japanese published work. J Dermatol 2014;41:845-9. [Google Scholar]
- 13.Liu SZ, Zhou X, Song A, Huo Z, Wang YP, Liu Y. Tumor-induced osteomalacia caused by a phosphaturic mesenchymal tumor of the femur. Chin Med J (Engl) 2019;132:2380-1. [Google Scholar]
- 14.Maehara J, Yamashita K, Hiwatashi A, Togao O, Kikuchi K, Matsumoto Y, et al. Primary phosphaturic mesenchymal tumour of the lumbar spine: Utility of 68Ga-DOTATOC PET/CT findings. BJR Case Rep 2016;2:20150497. [Google Scholar]
- 15.Munoz J, Ortega R, Celzo F, Donthireddy V. Tumour-induced osteomalacia. BMJ Case Rep 2012;2012:181. [Google Scholar]
- 16.Szumera-Ciećkiewicz A, Ptaszyński K, Pawełas A, Rutkowski P. Oncogenic osteomalacia associated with phosphaturic mesenchymal tumour, mixed connective tissue type of the knee. Pol J Pathol 2009;60:193-8. [Google Scholar]
- 17.Teramoto M, Kodama N, Kikkawa M, Nakamura A, Takemura Y, Ueba H, et al. Tumor-induced osteomalacia caused by a bone tumor in the ulna: A case report. JBJS Case Connector 2013;3:e126. [Google Scholar]
- 18.Kobayashi H, Ito N, Akiyama T, Okuma T, Kinoshita Y, Ikegami M, et al. Prevalence and clinical outcomes of hip fractures and subchondral insufficiency fractures of the femoral head in patients with tumour-induced osteomalacia. Int Orthop 2017;41:2597-603. [Google Scholar]
- 19.Ledford CK, Zelenski NA, Cardona DM, Brigman BE, Eward WC. The phosphaturic mesenchymal tumor: Why is definitive diagnosis and curative surgery often delayed? Clin Orthop Relat Res 2013;471:3618-25. [Google Scholar]
- 20.Liu S, Zhou X, Song A, Huo Z, Wang Y, Xia W, et al. Successful treatment of tumor-induced osteomalacia causing by phosphaturic mesenchymal tumor of the foot. Medicine (Baltimore) 2019;98:e16296. [Google Scholar]
- 21.Meng T, Zhou W, Li B, Yin H, Li Z, Zhou L, et al. En bloc resection for treatment of tumor-induced osteomalacia: A case presentation and a systematic review. World J Surg Oncol 2015;13. [Google Scholar]
- 22.Ogose A, Hotta T, Emura I, Hatano H, Inoue Y, Umezu H, et al. Recurrent malignant variant of phosphaturic mesenchymal tumor with oncogenic osteomalacia. Skeletal Radiol 2001;30:99-103. [Google Scholar]
- 23.Shenbaghavalli T, Harshavardhan JK, Menon PG. A rare case of phosphaturic tumor/oncogenic osteomalacia-diagnostic challenges and management algorithm. J Orthop Case Rep 2019;9:49-52. [Google Scholar]
- 24.Kim Y, Stein E, Remotti F, Lee FY. Tumor-induced osteomalacia secondary to a fibroblast growth factor 23-secreting phosphaturic mesenchymal tumor in the foot. JBJS Case Connect 2014;4:e22 [Google Scholar]
- 25.Kaur T, Rush ET, Bhattacharya RK. Phosphaturic mesenchymal heel tumor presenting with tumor-induced osteomalacia. 2018;5:e138-41. [Google Scholar]
- 26.Zuo Q, Wang H, Li W, Niu XH, Huang YH, Chen J, et al. Treatment and outcomes of tumor-induced osteomalacia associated with phosphaturic mesenchymal tumors: Retrospective review of 12 patients. BMC Musculoskelet Disord 2017;18:408. [Google Scholar]
- 27.Minisola S, Peacock M, Fukumoto S, Cipriani C, Pepe J, Tella SH, et al. Tumour-induced osteomalacia. Nat Rev Dis Primers 2017;3:17044. [Google Scholar]
- 28.Woo VL, Landesberg R, Imel EA, Singer SR, Folpe AL, Econs MJ, et al. Phosphaturic mesenchymal tumor, mixed connective tissue variant, of the mandible: Report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;108:925-32. [Google Scholar]
- 29.Seijas R, Ares O, Sierra J, Pérez-Dominguez M. Oncogenic osteomalacia: Two case reports with surprisingly diff erent outcomes. Arch Orthop Trauma Surg 2009;129:533-9. [Google Scholar]
- 30.Seufert J, Ebert K, Müller J, Eulert J, Hendrich C, Werner E, et al. Octreotide therapy for tumor-induced osteomalacia. N Engl J Med 2001;345:1883-8. [Google Scholar]
- 31.Shi Z, Deng Y, Li X, Li Y, Cao D, Coossa VS. CT and MR imaging features in phosphaturic mesenchymal tumor-mixed connective tissue: A case report. Oncol Lett 2018;15:4970-8. [Google Scholar]