Amyotrophic lateral sclerosis can be misdiagnosed as cervical or lumbar spine disorders, resulting in unnecessary surgeries and delayed treatment, which can adversely affect the prognosis.
Mr. Samuel Saucedo, Drexel University, College of Medicine, 2900 W Queen Ln, Philadelphia, Pennsylvania. E-mail: sts325@drexel.edu
Introduction: Almost 40% of patients who have been diagnosed with amyotrophic lateral sclerosis (ALS) may have been misdiagnosed. Some of these patients may have undergone surgical procedures to address symptoms that could have actually be early indications of ALS. Up to 40% of patients diagnosed with amyotrophic lateral sclerosis (ALS) have received an incorrect diagnosis, a number undergo surgical treatment for signs and symptoms that can be attributed to early manifestations of ALS. Initial presentation of ALS is elusive and is often mistaken for other disorders originating from the cervical spine such as cervical radiculopathy or myelopathy. Such incorrect diagnoses often display symptoms that fall within the scope of an orthopedic spine surgeon, who can remedy said diagnoses. Given that a diagnosis of ALS is grave, it is crucial to establish a definitive diagnosis quickly, without unnecessary surgery. The objective of this series is to highlight patients who were referred by other physicians for spine surgery to remedy potential side effects of cervical myelopathy but were ultimately diagnosed with ALS.
Case Report: Case 1: A 46-year-old Caucasian woman with carpal tunnel syndrome and cervical intervertebral disc degeneration. Case 2: A 77-year-old African American man with a history of arthritis, GERD, a herniated disc, claw hand, hypertension, prostate disease, and general weakness. Case 3: A 74-year-old Caucasian woman with a background history of hypertension, dyslipidemia, hypothyroidism, osteopenia, and foot drop.
Conclusion: In orthopedic spine surgery, ALS could be an easily misdiagnosed disease, which can be mistaken for cervical spondylosis, cervical radiculopathy, cervical myelopathy, lumbar radiculopathy, and lumbar myelopathy; it is of note to be aware of how ALS may initially present. It is imperative for the orthopedic spine surgeon to consider ALS with patients presenting with progressive unilateral/bilateral upper extremity weakness.
Keywords: Unnecessary surgery, cervical, myelopathy, amyotrophic lateral sclerosis, spine.
Up to 40% of patients diagnosed with amyotrophic lateral sclerosis (ALS) have received an incorrect diagnosis. Such incorrect diagnoses often overlap with problems in the purview of the spine surgeon such as radiculopathy or myelopathy. ALS prognosis is grave, with an average life expectancy of 2–5 years [1]. Unfortunately, many patients may lose valuable time chasing incorrect treatments, ultimately offering no benefit. ALS is a progressive, devastating, fatal motor neurodegenerative disease [2] with 90% of confirmed ALS cases appearing sporadic and 5–10% being familial [3]. The incidence of ALS prevalence in the United States is 5.2 cases/100,000 in the general United States population, with a higher prevalence in males [4]. ALS demonstrates a progressive motor weakness affecting the extremities. The initial symptoms of ALS may be vague, including weakness in the hand or arm and difficulty with simple tasks such as buttoning a shirt, writing, and using scissors. In some cases, symptoms present affecting a unilateral limb. People may experience clumsiness when ambulating, muscle spasticity, and difficulty chewing or swallowing as the disease progresses. When symptoms start in an upper or lower extremity, it is termed “limb onset.” When individuals first notice speech or swallowing problems, it is considered “bulbar onset” [5]. ALS may challenge the spine surgeon in the clinic as symptoms of ALS can mimic cervical disease. A definitive diagnosis requires a prolonged period, in part since ALS is primarily based on clinical assessment. A detailed medical history, physical examination, laboratory tests to exclude other diseases, electromyogram (EMG), nerve conduction study (NCS), and magnetic resonance imaging (MRI) are required (NIH). Cervical myelopathy is a clinical syndrome resulting from compression of the cervical spinal cord and may occur with degenerative disease of the cervical vertebrae [6]. Clinical features of cervical myelopathy may be similar to ALS, including weakness in the upper or lower extremities, loss of manual dexterity, gait imbalance, or loss of balance [7]. Similarly, with ALS, cervical myelopathy presents with both upper and lower motor neuron signs. Characteristic upper motor neuron symptoms occur below the level of injury, which includes weakness, hyperreflexia, spasticity, a positive Babinski or Hoffman test, and an ataxic broad-based gait. While, at the level of the lesion or lower, lower motor neuron symptoms are present and include atrophy, flaccidity, loss of muscle tone, and hyporeflexia [8]. In this case series, we present patients referred to the spine clinic with symptoms consistent with a diagnosis of ALS. After a thorough workup, the origins of their symptoms were determined and definitively diagnosed as ALS in each case. In orthopedic spine surgery, ALS could be an easily misdiagnosed disease. Therefore, we present our experiences in an orthopedic spine clinic where ALS was initially interpreted as peripheral neuropathies, cervical radiculopathy, cervical myelopathy, lumbar radiculopathy, or lumbar myelopathy.
Case 1
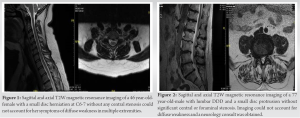
A 46-year-old woman with a background history of carpal tunnel syndrome (CTS) and cervical intervertebral disc degeneration was referred to the orthopedic spine for progressive left upper extremity weakness. The patient was 10 weeks post-CTR on her left side, which failed to alleviate her symptoms. The patient noted a 1.5-year history of left upper extremity weakness until she could no longer perform her duties as a hairstylist. There was no familial history of neurological disease. The patient had a previous EMG/NCS performed in October 2020, which revealed moderate-to-severe left CTS, mild right CTS, and mild cervical radiculopathy. Physical examination revealed bilateral hyperreflexia in upper and lower extremities (3+ biceps, brachioradialis, triceps, patellar, and Achilles), bilateral positive Hoffman sign, and bilateral upper and lower extremity weakness (deltoid 3/5 bilateral, biceps: Right 4/5, left 3/5, triceps: Right 4/5, left 3/5, wrist extensors: Left 4/5, wrist flexors: Left 4/5, hand intrinsic: Left 3/5, tibialis anterior: Right 4/5, left 4/5, extensor hallucis longus: Right 4/5, left 4/5). There was no sensation loss noted. Cervical MRI displayed degenerative disc disease present at C5-6, C6-7, and a C6-7-disc herniation resulting in moderate bilateral foraminal stenosis worsened on the left (Fig. 1). Considering the marked bilateral weakness of her upper extremities on the left more than right, hyperreflexia in both upper and lower extremities bilaterally, bilateral positive Hoffman sign, and upper extremity fasciculations, there was a suspicion for a motor neuron pathology. We referred the patient to neurology to obtain a repeat EMG/NCS and MRI of her brain. Repeat EMG in May 2021 demonstrated widespread denervation in both upper extremities, more significant on the patient’s left side than her right. Repeat EMG, including lower extremities, was abnormal as well. Neurology completed the neurology consult in June 2021, and the results stated that the differential diagnosis was unclear and may involve multifocal neuropathy vs. myopathic process vs. neuromuscular pathology. Neurology then referred the patient to another neurosurgeon. The patient had a telemedicine consultation with a neurosurgeon who recommended a C6-C7 arthroplasty. The patient returned to the clinic confused and anxious but was encouraged to follow up with a specialty motor neuron group. In August 2021, the patient followed up with a tertiary ALS referral center. By this time, her left had worsened, and she lost the ability to fasten buttons or write with a pen. The intrinsic left-hand muscle atrophied, and fasciculations were noted in both arms, more so in the left upper extremity. Weakness had developed in her lower left leg, which impacted her gait and balance, and provided a chronic opportunity for tripping. The neurological examination revealed fluent speech with trace spastic dysarthria. Cranial nerve examination displayed mild bifacial weakness on cheek puff and mildly slow side-to-side tongue movements. Motor examination revealed diminished muscle bulk of the left hand, and finger taps were markedly slow. The patient also displayed a slow, narrow-based gait and difficulty heel walking. After 2 years after the initial onset of symptoms: Asymmetric progressive limb weakness, subtle speech changes, mild bifacial weakness, and repeat EMG revealed a clinical diagnosis of ALS. The patient was started on riluzole and enrolled in clinical research trials.
Case 2
A 77-year-old man with a history of arthritis, GERD, a herniated disc, claw hand, hypertension, prostate disease, and general weakness was referred to orthopedic spine for back and leg pain related to lumbar degeneration. In the beginning of 2020, the patient noted that he had right leg weakness which progressed, and by May 2020, he required a cane to ambulate. Since May 2020, weakness in the right leg has progressed to his left leg and is now requiring the use of a walker to ambulate. In the past year, the patient has fallen upwards of 12 times and enrolled in physical therapy without any relief of his symptoms or strengthening of his leg. Physical therapy suggested the patient obtain an ankle-foot orthosis (AFO) for the recurrent right foot drop. After having the cast removed from his right arm, the patient experienced notable weakness, which was attributed to the presence of a CTR (carpal tunnel release) on that arm. The patient had a CTR on his right arm and once the cast was removed noted significant weakness. Physical examination grip strength was markedly decreased on the right vs. left (0 lbs. vs. 50 lbs.). Severe wasting of muscles was noted in both left and right upper extremities. The patient’s soft palate was collapsed. Strength was decreased bilaterally in the upper extremities (deltoid: Right 4/5, left 4/5, biceps: Right 3/5, left 4/5, triceps: Right 3/5, left 4/5, wrist extensors: Right 0/5, left 4/5). Lower extremity similarly showed a decrease in strength (iliopsoas: Right 4/5, left 4/5, quadriceps: Right 4/5, left 4/5, tibialis anterior: Right 0/5, left 4/5, peroneal: Right 4/5, left 4/5, hip abductors: Right 4/5, left 4/5, and gastrocnemius: Right 4/5, left 4/5). The patient also displayed hyperreflexia of the biceps: 3+ bilaterally, brachioradialis: 3+ bilaterally, and patellar: 3+ bilaterally, positive Hoffman side on the left hand. Sensation was intact. The patient was unable to walk for more than a few steps and difficulty raising from a chair. The patient’s gait was compromised and was unable to perform a heel walk, toe walk, or tandem gait. Plan films from February 2021 of the lumbar spine revealed multilevel lumbar degenerative disease, as well as Baastrup’s disease. In March 2021, MRI of the cervical spine and lumbar spine were done. The MRI image of the cervical spine showed signs of degenerative disc disease, along with moderate stenosis in the C5-C6 region. On the sagittal T2-weighted MRI of the cervical spine displayed degenerative disc disease, with moderate cervical stenosis noted at C5-C6. Sagittal MRI of the lumbar spine was significant for diffuse multilevel degenerative disc disease, multilevel posterior disc protrusions, and mild bilateral L5-S1 foraminal stenosis (Fig. 2) Considering the progressive weakness of the lower extremities, upper left extremity, and physical, the patient was referred to a neurologist at the UCSF Neurology ALS Center for an initial consultation and evaluation for a suspected motor neuron disease. EMG was performed in early May 2021 and reported chronic reinnervation and active denervation in the right cervical, thoracic, and lumbosacral myotomes, and a superimposed right mild median neuropathy at the wrist. In May 2021, a neurology consultation was conducted to asses the patient’s condition. The consultation involved a review of the patient’s medical history, a neurology examination, an electromyogram (EMG) test, negative neuroimaging results, and laboratory work. Based on these assessments, the patient was diagnosed with ALS. Neurology consult was done in May 2021 and determined with the patient’s history, neurological examination, EMG, negative neuroimaging, and laboratory work supported a diagnosis of ALS. The patient’s condition has worsened to the point where they cannot walk without the assistance of a walker. As a result, the patient needs a Hoyer lift, a power wheelchair, home care with physical and occupational therapy, and a PEG tube. Considering the patient has deteriorated significantly and can no longer ambulate without a walker, the patient requires a Hoyer lift, power wheelchair, homecare with PT/OT, and PEG tube. The patient was also started on riluzole and has been enrolled in an ALS support group.
Case 3
A 74-year-old woman with a background history of hypertension, dyslipidemia, hypothyroidism, osteopenia, and foot drop was referred to orthopedic spine surgery to evaluate her lumbar spine for right-sided foot drop. The patient was previously active but suffered a fall in July 2020 and “jammed” her right leg. She had suffered an 8–10-foot fall down a stairwell and started limping as a result. The patient reported increasing pain at night in her right foot. She was evaluated in physical therapy to try and improve her limp. During her physical therapy, she was walking her dog and stumbled over her dog, suffering a right foot fracture. In July 2021, the patient was prescribed an AFO boot that rose to her knee, potentially putting her at risk for peroneal compression at the fibular head. At this point, she was unsure if her continued limp was related to her initial injury or secondary to the heaviness of the boot. With the boot removed in August of 2021, the patient noted increasing falling and realized the absence of right-sided dorsiflexion or extension of her toes. On physical examination, the patient endorsed right leg pain, numbness, and tingling of the right foot and toes. The right foot was cool to the touch but had adequate perfusion, patent pedal, and posterior tibialis pulses. Grip strength was equal between both upper extremities (right: 49 lbs vs. left: 44 lbs), and bilateral hand tremors were noted. There is decreased upper extremity weakness bilaterally (deltoid: Right 4/5, left 4/5, biceps: Right 4/5, left 4/5, triceps: Right 4/5, left 4/5, hand intrinsic: Right 4/5, left 4/5). There is also a decrease in lower extremity motor weakness, right more than left (iliopsoas: Right 4/5, left 5/5, quadriceps: Right 4/5, left 5/5, tibialis anterior: Right 0/5. left 4/5, EHL: Right 0/5, left 4/5, peroneal: Right 3/5, left 5/5, hip abductors: Right 4/5, left 4/5, gastrocnemius: Right 3/5, left 5/5). There was no loss of sensation in the upper or lower extremities. No hyperreflexia was noted. The patient displayed a steppage gait and was unable to perform a heel walk, toe walk, or tandem gait. Previous sagittal MRI displayed multilevel degenerative disc disease with Modic endplate changes. A small disc protrusion was noted at L1-L2 and L3-4, and no significant central neuroforaminal stenosis was noted. Plain lumbar films noted degenerative disc disease at the L3-L4 level. A previous DEXA scan revealed osteopenia (lumbar spine T-score: −1.8, left femur T-score: −1.5, right femur T-score: −1.6). Given the history of progressive weakness, mild hyperreflexia, and tremors in the upper limbs, a motor neuron disease was highly suspected. We referred the patient to a neurologist for an EMG. The EMG reported evidence of severe asymmetric sensorimotor peripheral polyneuropathy with axonal and demyelinating characteristics in the upper and lower extremities bilaterally with denervation present in the lower extremities, right side greater than her left side. The EMG results showed signs of a median neuropathy at both writs, which could indicate moderate to severe carpal tunnel syndrome. It was challenging to diagnose, but the possibility of CTS cannot be rules out. The EMG also reported evidence of a superimposed median neuropathy at the wrists bilaterally. Although difficult to identify but potentially in the moderate-to-severe range for CTS. Considering the laterality of demyelination of the lower extremities, right side greater than left side, of the EMG, the patient was referred to neurology for a definitive diagnosis. The neurologist consulted was performed over Zoom and noted no history of fasciculations, atrophy, bulbar signs, dyspnea, weight loss, or any other focal weakness. There is sensory loss on the dorsolateral aspect and nocturnal paresthesias in bilateral thenar eminence Neurology examination noted slow finger and foot taps, marked right ankle dorsiflexion, toe extension, and lateral ankle rotation. Overall localization of her right-sided foot drop could include peroneal neuropathy at the fibular head, L5 radiculopathy, and sciatic neuropathy instead of a motor neuron disease. Neurology recommended an in-person evaluation, EMG/NCS to further evaluate for motor neuron disease. Overall localization of her right-sided foot drop could include peroneal neuropathy at the fibular head, L5 radiculopathy, and sciatic neuropathy instead of a motor neuron disease. Neurology recommends an in-person evaluation, EMG/NCS to further evaluate for motor neuron disease. The current recommendation from neurology is continued use of AFO boot and physical therapy and recommended conservative therapy with wrist splints for suspected CTS.
ALS has a poor prognosis with only a 2–5-year life expectancy, and unfortunately, many patients will spend that time chasing incorrect diagnoses [1]. For this reason, it is advantageous to properly diagnose ALS to give the maximum available time for a patient to start treatment, prevent unnecessary interventions, manage expectations, etc. A diagnosis of ALS requires following the El Escorial criteria, established in 1990 at the annual world federation of neurology. The requirements require (1) signs of degeneration of lower motor neurons, (2) signs of degeneration of upper motor neurons, (3) progressive spread of signs within a region to other regions, and (4) absence of electrophysiological, pathological, and neuroimaging evidence of other disease processes [9]. Once the criteria have been satisfied, a patient can be diagnosed with ALS definitively. The timeline to diagnosis ALS can range anywhere from 9.3 months to 11.3 months in total. Not only does the initial workup potentially last 11 months or longer for a diagnosis of ALS, but also misdiagnosing lengthens the time from the initial presentation. Paganoni [10] reported that 52% of the patients included in their population study had received a misdiagnosis before ALS was confirmed. A delayed diagnosis may have significant mental health medical side effects and potentially hinder patients from enrollment in clinical research trials. The most common misdiagnoses are neuropathy, spine, vascular, neurodegenerative, and NMJ. Among misdiagnosed patients, 21% of patients had inappropriate surgeries for symptoms that were later relieved as early manifestations of ALS [11]. Rowland et al. [12] reported that 5% of their retrospective ALS cohort had undergone cervical or lumbar laminectomies early in the course of the disease. Similarly, Yoshor et al. [13] reported that 4.2% of unrecognized ALS patients underwent spinal decompression surgery. Of those patients included in the Srinivasan study, inappropriate spine surgery occurred in 14 patients (3 cervical, 11 lumbar) and 5 CTR (carpal tunnel release) surgeries. In all unnecessary surgical cases, the symptoms that prompted an intervention did not resolve and were classified as initial manifestations of ALS. Therefore, it is vital to the orthopedic spine surgeon that misdiagnosis of ALS could result in costly unnecessary surgeries. In the first case presented in this series, the patient was initially evaluated for weakness in their dominant hand and had an EMG performed that suggested CTS. The patient underwent a CTR, which did not relieve their symptoms. With the benefit of hindsight, it would be appropriate to attribute the left upper extremity’s progressive weakness as an initial manifestation of ALS. Another potential red herring in case 1 is her 20-year career as a hairstylist, which has shown to have a 75% increased frequency of CTS over the unemployed [14]. Paganoni et al. [10] reported that patients who had an initial misdiagnosis saw an average of three different physicians throughout their workup, which contributes to the increasing diagnostic timeline of ALS. Being attended to by other physicians can also lead to multiple alternative diagnoses, which was the case for the patients referred to our orthopedic spine clinic. In case 1 of this series, the patient saw a total of 7 other physicians before they had a diagnosis. The author, Orthopaedic spine surgeon, a consulting neurosurgeon, and two neurologists also evaluated the patient. The author, orthopedic spine, saw this patient, a consulting neurosurgeon, and two neurologists also saw them. The initial consulting neurologist could not suggest a diagnosis of ALS or elicit a differential diagnosis that included ALS. Not only did that lead to an increased diagnostic timeline, but also it led to a virtual neurosurgical consult in which they recommended an inappropriate C6-C7 arthroplasty. Although virtual consults are potent tools to expand treatments, we also suggest a confirming physical examination diagnosis before offering a surgical intervention. In orthopedic spine surgery, ALS could be an easily misdiagnosed disease, which can be mistaken for cervical spondylosis, cervical radiculopathy, cervical myelopathy, lumbar radiculopathy, and lumbar myelopathy; it is of note to be aware of how ALS may initially present. It is imperative for the orthopedic spine surgeon to consider ALS with patients presenting with progressive unilateral/bilateral upper extremity weakness. The patients presented in this case series were all desperate for treatment (even more surgery if necessary). Furthermore, they were referred by doctors who also requested surgery to be performed. It is critical for the spine surgeon to remain sober in such situations, examine the facts, and only offer surgery if it is clinically necessary or is the last line of treatment available.
Forty percentage of patients diagnosed with ALS have received an incorrect diagnosis, and such misdiagnoses often overlap with problems in the purview of the spine surgeon, such as radiculopathy or myelopathy. A definitive diagnosis of ALS requires a prolonged period and a detailed medical history, physical examination, laboratory tests to exclude other diseases, EMG, NCS, and MRI. The initial symptoms of ALS may be vague, including weakness in the hand or arm, and symptoms may present affecting a unilateral limb. The diagnosis of ALS is crucial to give the maximum available time for a patient to start treatment, prevent unnecessary interventions, and manage expectations. The most common misdiagnoses are neuropathy, spine, vascular, neurodegenerative, and NMJ, and misdiagnosis could result in costly unnecessary surgeries. Therefore, the orthopedic spine surgeon must be vigilant in the differential diagnosis and not confuse ALS symptoms with cervical or lumbar spine disease.
Neurological symptoms are very common and should be investigated thoroughly, before referring to a surgeon for definitive treatment.
References
- 1.Byrne S, Jordan I, Elamin M, Hardiman O. Age at onset of amyotrophic lateral sclerosis is proportional to life expectancy. Amyotroph Lateral Scler Frontotemporal Degener 2013;14:604-7. [Google Scholar]
- 2.Logroscino G, Traynor BJ, Hardiman O, Chiò A, Mitchell D, Swingler RJ, et al. Incidence of amyotrophic lateral sclerosis in Europe. J Neurol Neurosurg Psychiatry 2010;81:385-90. [Google Scholar]
- 3.Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med 2001;344:1688-700. [Google Scholar]
- 4.Mehta P, Antao V, Kaye W, Sanchez M, Williamson D, Bryan L, et al. Prevalence of amyotrophic lateral sclerosis - United States, 2010-2011. MMWR Morb Mortal Wkly Rep 2014;63:1-13. [Google Scholar]
- 5.Amyotrophic Lateral Sclerosis (ALS) Fact Sheet National Institute of Neurological Disorders and Stroke. Available from: https://www.ninds.nih.gov/disorders/patient-caregiver-education/fact-sheets/amyotrophic-lateral-sclerosis-als-fact-sheet [Last accessed on 2022 May 01]. [Google Scholar]
- 6.TA, Coughlin, and Klezi Z. “Focus on Cervical Myelopathy .” Hudson Spine, 4 June 2016, https://hudsonspine.com/wp-content/uploads/2016/10/cervical-myelopathy-10-26-2016.pdf. [Google Scholar]
- 7.Badhiwala JH, Ahuja CS, Akbar MA, Witiw CD, Nassiri F, Furlan JC, et al. Degenerative cervical myelopathy - update and future directions. Nat Rev Neurol 2020;16:108-24 [Google Scholar]
- 8.Yamada M, Furukawa Y, Hirohata M. Amyotrophic lateral sclerosis: Frequent complications by cervical spondylosis. J Orthop Sci 2003;8:878-81. [Google Scholar]
- 9.Diagnosis - Amyotrophic Lateral Sclerosis (ALS) - Diseases. Muscular Dystrophy Association; 2015. Available from: https://www.mda.org/disease/amyotrophic-lateral-sclerosis/diagnosis [Last accessed on 2022 May 01]. [Google Scholar]
- 10.Paganoni S, Macklin EA, Lee A, Murphy A, Chang J, Zipf A, et al. Diagnostic timelines and delays in diagnosing amyotrophic lateral sclerosis (ALS). Amyotroph Lateral Scler Frontotemporal Degener 2014;15:453-6. [Google Scholar]
- 11.Srinivasan J, Scala S, Jones HR, Saleh F, Russell JA. Inappropriate surgeries resulting from misdiagnosis of early amyotrophic lateral sclerosis. Muscle Nerve 2006;34:359-60. [Google Scholar]
- 12.Rowland LP. Diagnosis of amyotrophic lateral sclerosis. J Neurol Sci 1998;160:S6-24. [Google Scholar]
- 13.Yoshor D, Klugh A, Appel SH, Haverkamp LJ. Incidence and characteristics of spinal decompression surgery after the onset of symptoms of amyotrophic lateral sclerosis. Neurosurgery 2005;57:984-9. [Google Scholar]
- 14.Demiryurek BE, Gündoğdu AA. Prevalence of carpal tunnel syndrome and its correlation with pain amongst female hairdressers. Int J Occup Med Environ Health 2017;31:1-7. [Google Scholar]