To highlight the importance of considering pyoderma gangrenosum as a potential etiology for non-healing wounds and to maintain a low threshold for skin biopsy and early interdisciplinary care to expedite care and provide effective treatment.
Dr. Grayson A. Domingue, Department of Orthopaedic Surgery, University of Oklahoma Health Sciences Center, 800 Stanton L Young Boulevard, Andrews Academic Tower 3400, Oklahoma City, Oklahoma 73104, United States. E-mail: grayson-domingue@ouhsc.edu
Introduction: Pyoderma gangrenosum (PG) is a skin condition driven by neutrophil activation resulting in painful ulcers with undermining borders and surrounding erythema. This can be seen, although rarely, post-traumatically. It has been reported in the setting of orthopedic trauma with only 31 cases reported in English literature after orthopedic surgery.
Case Report: A 20-year-old Caucasian female presented with multisystem trauma and multiple orthopedic injuries following motor vehicle collision. After fixation of orthopedic injuries, within 1 week post-operatively, the patient began to show signs of wound breakdown characterized by apparent purulence and skin necrosis at surgical sites and subsequently at additional non-surgical sites on bilateral lower extremities. After the failure of aggressive debridement and negative cultures, skin biopsy revealed post-traumatic PG. After diagnosis and treatment with corticosteroid therapy, the patient promptly recovered with the resolution of systemic and musculoskeletal manifestations.
Conclusion: Post-traumatic PG should be considered a potential etiology in non-healing wounds with negative cultures. A low threshold for skin biopsy and interdisciplinary involvement should be maintained to expedite diagnosis and guide treatment.
Keywords: Trauma, infection, inflammatory response, pyoderma gangrenosum.
Pyoderma gangrenosum (PG) is a skin condition driven by neutrophil activation resulting in painful ulcers with undermining borders and surrounding erythema [1]. PG is often thought to be immunologic in nature and is commonly associated with systemic disease. Upregulation of several key proinflammatory and chemotactic factors within the ulcers have been identified including interleukin-17, tumor necrosis factor a (TNF-a), IL-8, IL-6, IL-23, and IL-1 [1]. The lower legs are the most common location where PG arises, and these lesions are often precipitated or worsened by traumatic events, a phenomenon known as “pathergy” [2]. It is common for PG to be misdiagnosed as a simple non-healing ulcer or a necrotizing infection if acute in presentation [3]. These patients will subsequently undergo debridement, which leads to massive deterioration and spreading of the lesions through this pathergic response. This highlights the difficulty in prompt diagnosis of this condition, especially in the practice of orthopedic surgeons where they are more commonly dealing with the PG mimickers such as infection, ulcers, and necrotizing fasciitis. To the best of our knowledge, there has been a total of 35 reported cases in the literature of PG following orthopedic or trauma surgery, with only 7 of those cases specifically covering fractures of the extremities. We give an account for a polytraumatized individual following motor vehicle collision (MVC) who developed PG-mimicking post-operative infection following initial stabilization efforts in the operating room. This patient was informed that data concerning the case would be submitted for publication, and she provided consent.
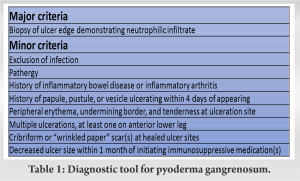
A 20-year-old female presented with multisystem trauma and multiple orthopedic injuries following MVC. Most notably, she presented with fractures of the iliac wing, bilateral tibias (right open), open talus fracture dislocation, and humerus. The patient subsequently underwent urgent surgical stabilization of these injuries. She tolerated the procedures well and initially showed no signs of wound compromise. Within 1 week post-operatively, the patient began to show signs of wound breakdown characterized by apparent purulence and skin necrosis at surgical sites and subsequently at additional non-surgical sites on bilateral lower extremities (Fig. 1). Concomitantly, the patient’s vital signs began to deteriorate with persistently elevated temperatures rising up to 40.2°C, tachycardia to 150, and leukocytosis to 50,000. Due to high clinical suspicion of surgical site infection, the patient was started on broad-spectrum antibiotics and underwent aggressive, serial surgical debridement of the affected wounds, as well as surgical removal of hardware and placement of antibiotic cement. Despite these efforts, the patient showed no clinical improvement with apparent worsening (Fig. 2). 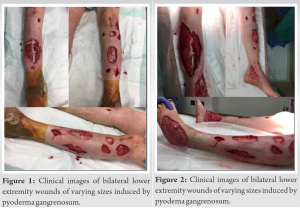
Classically, the presentation of postoperative or posttraumatic PG is erythema of skin surrounding the site of surgery and pain out of proportion 1 week following surgery [4]. Tolkachjov et al. found that it took 11 days on average for signs and symptoms of pain and wound dehiscence [5]. The wound classically will have raised violaceous, undermined borders. Another clue can be the presence of fever, leukocytosis, and elevated inflammatory markers. However, this is more commonly associated with infection, which explains why one study found 56% of patients with PG had undergone antibiotic therapy and 61% underwent debridement [5]. Ebrad et al. conducted a systematic review on 30 patients with post-operative PG following orthopedic surgery including total joint replacement, arthroscopy, trauma, spine, foot, and hand surgery [6]. Despite the majority of documented cases being associated with total joint replacement and trauma surgery, their review shows PG can arise in a wide variety of orthopedic procedures. Thus, far in the literature, there are no established criteria for diagnosis. Recently, Maverakis et al. proposed a diagnostic criterion based on expert opinion from various countries, requiring one major and four minor criteria to establish diagnosis (Table 1) [7]. This method of diagnosis has not been widely adopted to this point, but it allows providers an alternate route of diagnosis compared to the traditional method being diagnosis of exclusion. The histology of skin biopsies is an important aspect in evaluating patients where PG may be suspected. The main value of biopsy is to exclude alternate causes of skin ulceration and allow specimens to be sent for bacterial, mycobacterial, and fungal cultures. The biopsy needs to include both the active ulcer border and also extend deeper into the subcutaneous tissues [1]. Histology may show ulceration of the epidermis and dermis along with dense neutrophilic infiltrate, neutrophilic pustules, and abscess formation [8]. These findings are not specific and may differ contingent upon the PG variant, and it is important to expect the biopsy will cause an enlargement of the ulcer along with the potential spread of the ulcer due to the pathergic nature of the disease. Consultation of dermatology can be an additional route of assistance in evaluating these lesions. As for treatment, most recommendations consist of medical treatment with systemic steroids or cyclosporine, with clinical improvement often seen in 24 h [9]. Ebrad et al. found all 30 cases of PG in their systematic review had utilized systemic corticosteroid therapy with wound healing in 1–9 months [6]. This highlights that up to this point in the literature, high-dose corticosteroids are the mainstay of treatment. Long-term steroids bring the risk of several different adverse side effects, so these are often weaned once clinical improvement is seen. Supplementation with other immunosuppressive agents such as tacrolimus, TNF-a, IL-b antagonists, mycophenolate mofetil, azathioprine, and polyvalent immunoglobulins which may be considered to completely eradicate the PG. However, high-level evidence showing the efficacy of these treatments is not present in the literature [10, 11]. When it comes to surgical treatment of these lesions, it is only helpful in the setting of controlled PG to surgically remove necrotic tissue either to decrease the risk of infection or prepare for skin graft [12]. It is important to note that one of the most critical components is to get a prompt and accurate diagnosis of PG to avoid multiple debridements and secondary surgeries leading to increased patient morbidity.
Due to its rarity, the higher likelihood of infection given the presenting symptoms, and lack of understanding of PG, delays in diagnosis of this pathology often result. For the patient with post-surgical wound breakdown and systemic inflammatory response, infection remains to be the most likely diagnosis. However, in the setting of negative cultures and persistent clinical worsening despite aggressive debridement and antibiotic coverage, one should consider other potential etiologies such as autoimmune, rheumatologic, and dermatologic conditions such as PG. We believe education and the use of multidisciplinary teams can be tools to increase awareness of this problem. Furthermore, surgical teams should keep PG on the differential for non-healing wounds, especially in recently traumatized individuals.
Surgical teams should keep PG on the differential for non-healing wounds, especially in recently traumatized individuals. A lower threshold to engage consultants and perform biopsy can expedite appropriate care for these patients.
References
- 1.George C, Deroide F, Rustin M. Pyoderma gangrenosum-a guide to diagnosis and management. Clin Med (Lond) 2019;19:224-8. [Google Scholar]
- 2.Sassolas B, Le Ru Y, Plantin P, Lair G, Dupre PF, Cochard G, et al. Pyoderma gangrenosum with pathergic phenomenon in pregnancy. Br J Dermatol 2000;142:827-8. [Google Scholar]
- 3.Bates T, Sheean AJ, Kao E, Bandino JP, Lynch TB, Lybeck D. Fulminant pyoderma gangrenosum after outpatient knee arthroscopy. J Am Acad Orthop Surg Glob Res Rev 2021;5:e21.00006. [Google Scholar]
- 4.Zuo KJ, Fung E, Tredget EE, Lin AN. A systematic review of post-surgical pyoderma gangrenosum: Identification of risk factors and proposed management strategy. J Plast Reconstr Aesthet Surg 2015;68:295-303. [Google Scholar]
- 5.Tolkachjov SN, Fahy AS, Wetter DA, Brough KR, Bridges AG, Davis MD, et al. Postoperative pyoderma gangrenosum (PG): The Mayo Clinic experience of 20 years from 1994 through 2014. J Am Acad Dermatol 2015;73:615-22. [Google Scholar]
- 6.Ebrad S, Severyns M, Benzakour A, Roze B, Derancourt C, Odri GA, et al. Pyoderma gangrenosum after orthopaedic or traumatologic surgery: A systematic revue of the literature. Int Orthop 2018;42:239-45. [Google Scholar]
- 7.Maverakis E, Ma C, Shinkai K, Fiorentino D, Callen JP, Wollina U, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: A delphi consensus of international experts. JAMA Dermatol 2018;154:461-6. [Google Scholar]
- 8.Weedon D, Johnston R. Weedon’s Skin Pathology Essentials. United Kingdom: Churchill Livingstone; 2012. [Google Scholar]
- 9.Chow RK, Ho VC. Treatment of pyoderma gangrenosum. J Am Acad Dermatol 1996;34:1047-60. [Google Scholar]
- 10.Alavi A, French LE, Davis MD, Brassard A, Kirsner RS. Pyoderma gangrenosum: An update on pathophysiology, diagnosis and treatment. Am J Clin Dermatol 2017;18:355-72. [Google Scholar]
- 11.Partridge AC, Bai JW, Rosen CF, Walsh SR, Gulliver WP, Fleming P, et al. Effectiveness of systemic treatments for pyoderma gangrenosum: A systematic review of observational studies and clinical trials. Br J Dermatol 2018;179:290-5. [Google Scholar]
- 12.Reichrath J, Bens G, Bonowitz A, Tilgen W. Treatment recommendations for pyoderma gangrenosum: An evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol 2005;53:273-83. [Google Scholar]