The subacromial balloon is a minimally invasive novel technology that can aid in the management of massive irreparable rotator cuff tears and can provide an easy and speedy rehabilitation and recovery.
Dr. Mohamad Y. Fares, Division of Shoulder and Elbow Surgery, Rothman Orthopaedic Institute, Philadelphia, Pennsylvania, USA. E-mail: mohamadfaresmd@gmail.com
Introduction: Massive irreparable rotator cuff tears are challenging pathologies with many treatment modalities and therapeutic approaches. In patients with certain indications, the subacromial balloon spacer can effectively alleviate pain and improve function, in a manner that may be superior to other management options.
Case Report: We report the case of a 64-year-old active male who had previously underwent a subacromial balloon placement in his right shoulder and an arthroscopic rotator cuff repair procedure on his left shoulder. He later presented with persistent pain and disability in his left shoulder and opted to undergo a second subacromial balloon placement on his left side. To the best of our knowledge, this is the first case of bilateral subacromial balloon placement procedure in the literature.
Conclusion: The subacromial balloon is a safe treatment modality for irreparable rotator cuff tears, and its introduction into bilateral shoulders can provide an easier recovery and rehabilitation when compared to other more invasive procedures.
Keywords: Subacromial balloon, rotator cuff, shoulder, partial cuff repair, interpositional balloon.
Massive tears of the rotator cuff are debilitating and painful injuries with no clear-cut management guidelines or conclusive therapeutic approaches [1]. Treatment is often catered and tailored according to the individual case and its characteristics, and as such, prognostic outcomes vary widely between different patients [2]. Options include rotator cuff repair, tendon transfer, superior capsular reconstruction, and reverse shoulder arthroplasty, with each carrying a prominent risk of questionable clinical and functional outcomes [3]. In the recent years, a new innovative treatment option emerged, with great potential for solving the clinical issues associated with a specific population of rotator cuff tears. The InSpace biodegradable balloon is a device that is used to space out the distance between the humeral head and the acromion, thereby decreasing the superior migration of the humeral head and alleviating friction and impingement [4, 5]. The balloon can be inserted through an arthroscopic approach with much surgical ease and convenience and has shown great promise and benefit in a select group of patients [5]. Inclusion criteria include: a massive rotator cuff tear, normal deltoid function, an intact subscapularis, and an ability to elevate the arm to 90°. Exclusion criteria include severe glenohumeral arthritis, deltoid palsy, and a reparable rotator cuff tear [6, 7]. In light of the variable results of the traditional options for rotator cuff repair, exploring the use of balloon spacers in irreparable rotator cuff patients can give us additional insight into the diagnostic and prognostic features of this novel treatment option. In this work, we report the case of a patient who had presented for a balloon spacer in his right rotator cuff in the past and now opted to repeat the procedure for his previously-repaired now-retorn left rotator cuff. Accordingly, and to our knowledge, this is the first report of a patient having bilateral balloon spacers for rotator cuff tears in both shoulders.
This is the case of a 64-year-old male mason who presented with left shoulder pain and disability. The patient underwent two procedures 10 months before presentation: a revision rotator cuff repair in his left shoulder and placement of a subacromial interpositional balloon spacer in his right shoulder. He continued to have significant pain in his left shoulder and felt a discrepancy between his two shoulders in terms of strength and function, favoring the right side. He presented for a discussion of options regarding treatment for his left shoulder. He denied any traumatic incident since his last procedures. He is taking opioid medication to control his shoulder pain. He has a history of hypertension and hypercholesterolemia, along with a surgical history that included a cerebral bypass and a cardiac stent placement procedure, for which he takes aspirin and ticagrelor. He also had a recent hemithyroidectomy for a thyroid cyst with an uneventful post-operative course. The patient has no history of inflammatory or rheumatologic diseases. He has a strong build with a height of 185 cm and weight of 101 kg. On physical examination, the patient demonstrated good cervical spine mobility with only mild limitations. The left shoulder showed well healing scars from his previous procedure, with mild muscular atrophy. Range of motion testing showed 140° of forward elevation, 90° of abduction, 30° of external rotation, and passive internal rotation reaching upper lumbar spine. Strength testing showed a score of 5 on forward elevation, 4+ on external rotation, and 5 on internal rotation. The patient had a negative belly press test and was neurovascularly intact. The patient rated his right shoulder to be in a significantly better state than his left shoulder and contemplated the possibility of undergoing a subacromial balloon placement in his left side as well to treat his pain. Magnetic resonance imaging (MRI) of the left shoulder demonstrated a large retear of the previously repaired rotator cuff, mainly at the supraspinatus tendon and extending into his infraspinatus tendon (Fig. 1). 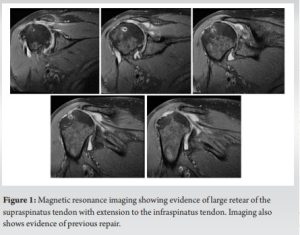
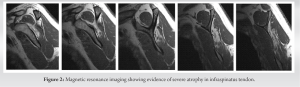
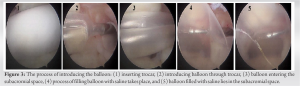
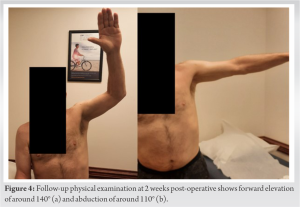
The optimal treatment of irreparable rotator cuff tears has been debated for many years, with consensus often directed toward a patient-centered approach, where procedures are catered to the individual patient [8]. The subacromial balloon spacer has been introduced as a novel surgical technique in shoulder surgery with great therapeutic potential in the domain of irreparable rotator cuff tears [9]. This device is made from a biodegradable and bioabsorbable polymer, with properties that ensure both the elasticity and strength required to be introduced in the subacromial space and alleviate any concurrent impingement [10, 11, 12, 13]. Evaluation of the safety profile of the device showed no evidence of systemic toxicity or chronic body reaction after its introduction into the body [10]. As a result, and over the recent years, the balloon has been considered a viable and safe option for many rotator cuff patients, whose presentation fits the indications and contraindications of its placement. The balloon offers a solution to a select group of irreparable rotator cuff patients with specific indications. Indications for a subacromial balloon mainly include a torn and irreparable supraspinatus and infraspinatus tear, with an intact and functioning subscapularis and teres minor, to preserve coupling of internal and external rotation [14]. Exclusion criteria include glenohumeral arthropathy, joint infection, pseudoparalysis, axillary nerve palsy, and allergy to device material [14]. In our case, the patient presented with an irreparable rotator cuff tendon, localized mainly at the level of the supraspinatus and extending into the infraspinatus. He presented with an intact subscapularis, and an adequate range of motion, thereby negating any possible presentation of pseudoparalysis. He had no active joint infection, and had low/intermediate arthritic changes, and accordingly, he was deemed a good candidate for the placement of subacromial balloon. The biomechanical theory behind the subacromial balloon relies on its ability to reduce subacromial friction by depressing the humeral head inferiorly to increase the acromiohumeral interval [10]. By doing so, and given the presence of intact subscapularis and teres minor in our patient, the balloon is able to restore proper kinematics in a shoulder with deficient rotator cuff and can consequently improve pain and function [10]. The balloon changes the center of rotation in the glenohumeral joint and increases of efficiency of other surrounding musculature to compensate for the defect in the supraspinatus and infraspinatus tendons [14]. In that study, patients noted significant improvement in daily activities and range of motion as early as 6 weeks and 8 weeks post-procedure, respectively [15]. Another study performed a multi-center randomized clinical trial to compare the outcomes of subacromial balloon placement to that of arthroscopic partial repair, in patients with massive rotator cuff tears and an intact subscapularis [16]. The subacromial balloon group noted an earlier functional recovery and pain relief when compared to the arthroscopic partial repair group [16]. These studies complemented the presentation of our patient, who had previously undergone a subacromial balloon placement in his right shoulder and an arthroscopic repair in his affected left shoulder, but still felt that his left shoulder was inferior to his right, with regard to pain and function. The patient opted for a second subacromial balloon in his right shoulder, which may be reflective of the positive attitude exhibited by patients toward this procedure, especially when considering its minimal invasiveness, faster pain relief, and its relatively easier recovery and rehabilitation. Additional advantages to the subacromial balloon placement procedure include the significantly decreased surgical time [16]. The procedure for our patient required around 20 min from skin incision till wound closure, thereby minimizing risks of prolonged anesthesia. Given that no arthroscopic repair was conducted in this case, the patient was instructed to be in a sling for 10 days and start physical therapy at around 2 weeks post-procedure. At 2 months post-operative, the patient was satisfied and had been undergoing an uneventful recovery.
Treating massive irreparable rotator cuff tears is a challenge in the world of shoulder surgery, with many debates over the efficacy of one treatment option over the other. The subacromial balloon spacer offers a novel, efficacious, and simple solution to a select group of patients who fit the indications criteria. These criteria include the presence of a massive irreparable rotator cuff tear with an intact subscapularis and an intact teres minor. These also include the absence of severe glenohumeral arthropathy, active infection, or joint pseudoparalysis. The subacromial balloon spacer has been proven to restore function and alleviate pain in these patients with minimal surgical time, allowing it to be a very beneficial tool for many surgeons and rotator cuff patients worldwide. Future research should explore the long-term outcomes of the subacromial balloon, to gain more insights onto its longevity, efficacy, and prognosis.
- Massive irreparable rotator cuff tears are challenging pathologies with diverse treatment modalities
- The subacromial balloon spacer is a novel technology that is inserted into the glenohumeral joint to increase the distance between the humeral head and the acromion
- Through spacing out acromiohumeral distance and altering tension of adjacent musculature, the balloon alleviates impingement and pain in shoulder joint and improves function
- The balloon is safe and its insertion into bilateral shoulders does not result in significant discomfort, as may be seen with other more invasive procedures.
References
- 1.Schumaier A, Kovacevic D, Schmidt C, Green A, Rokito A, Jobin C, et al. Defining massive rotator cuff tears: A Delphi consensus study. J Shoulder Elbow Surg 2020;29:674-80. [Google Scholar]
- 2.Burnier M, Elhassan BT, Sanchez-Sotelo J. Surgical management of irreparable rotator cuff tears: What works, what does not, and what is coming. J Bone Joint Surg Am 2019;101:1603-12. [Google Scholar]
- 3.Kucirek NK, Hung NJ, Wong SE. Treatment options for massive irreparable rotator cuff tears. Curr Rev Musculoskelet Med 2021;14:304-15. [Google Scholar]
- 4.Vecchini E, Gulmini M, Peluso A, Fasoli G, Anselmi A, Maluta T, et al. The treatment of irreparable massive rotator cuff tears with inspace balloon: Rational and medium-term results. Acta Biomed 2022;92:e2021584. [Google Scholar]
- 5.Senekovic V, Poberaj B, Kovacic L, Mikek M, Adar E, Markovitz E, et al. The biodegradable spacer as a novel treatment modality for massive rotator cuff tears: A prospective study with 5-year follow-up. Arch Orthop Trauma Surg 2017;137:95-103. [Google Scholar]
- 6.Lobao MH, Canham RB, Melvani RT, Abboud JA, Parks BG, Murthi AM. Biomechanics of biodegradable subacromial balloon spacer for irreparable superior rotator cuff tears: Study of a cadaveric model. J Bone Joint Surg Am 2019;101:e49. [Google Scholar]
- 7.Stewart RK, Kaplin L, Parada SA, Graves BR, Verma NN, Waterman BR. Outcomes of subacromial balloon spacer implantation for massive and irreparable rotator cuff tears: A systematic review. Orthop J Sports Med 2019;7:2325967119875717. [Google Scholar]
- 8.Novi M, Kumar A, Paladini P, Porcellini G, Merolla G. Irreparable rotator cuff tears: Challenges and solutions. Orthop Res Rev 2018;10:93-103. [Google Scholar]
- 9.Savarese E, Romeo R. New solution for massive, irreparable rotator cuff tears: The subacromial “biodegradable spacer”. Arthrosc Tech 2012;1:e69-74. [Google Scholar]
- 10.Viswanath A, Drew S. Subacromial balloon spacer – Where are we now? J Clin Orthop Trauma 2021;17:223-32. [Google Scholar]
- 11.Basu A, Haim-Zada M, Domb AJ. Biodegradable inflatable balloons for tissue separation. Biomaterials 2016;105:109-16. [Google Scholar]
- 12.Haim Zada M, Kumar A, Elmalak O, Markovitz E, Icekson R, Machlev E, et al. Biodegradable implantable balloons: Mechanical stability under physiological conditions. J Mech Behav Biomed Mater 2019;100:103404. [Google Scholar]
- 13.Haim Zada M, Kumar A, Elmalak O, Markovitz E, Icekson R, Domb AJ. In vitro and in vivo degradation behavior and the long-term performance of biodegradable PLCL balloon implants. Int J Pharm 2020;574:118870. [Google Scholar]
- 14.Horneff JG, Abboud JA. Balloon arthroplasty: Indications, technique, and European outcomes. Ann Joint 2018;3. [Google Scholar]
- 15.Senekovic V, Poberaj B, Kovacic L, Mikek M, Adar E, Dekel A. Prospective clinical study of a novel biodegradable sub-acromial spacer in treatment of massive irreparable rotator cuff tears. Eur J Orthop Surg Traumatol 2013;23:311-6. [Google Scholar]
- 16.Verma N, Srikumaran U, Roden CM, Rogusky EJ, Lapner P, Neill H, et al. InSpace implant compared with partial repair for the treatment of full-thickness massive rotator cuff tears: A multicenter, single-blinded, randomized controlled trial. J Bone Joint Surg Am 2022;104:1250-62. [Google Scholar]







