Unexpected complication like neurological deterioration can happen in a chronically stenotic canal and hence, a proper pre-operative counseling can reduce the stress for both the surgeon, the patient, and his family.
Dr. Shashi Ranjan, Department of Orthopedics, Agartala Government Medical College, Agartala, Tripura, India. Email: shashi26492@gmail.com
Introduction: A new neurologic deficit after spine surgery is always the biggest surgeon’s nightmare. Worsening of neurology post-operatively in the absence of obvious per operative injury and with no extrinsic cause, the deficit is attributable to be caused by reperfusion injury of the spinal cord called as white cord syndrome (WCS). Hereby, we report 1-year follow-up of a case attributed as WCS after anterior cervical corpectomy with complete recovery.
Case Report: A 64-year-old female patient presented with C5 - C6 tubercular lesion with extradural compression with ASIA C grade, treated with C5 - C6 corpectomy with harm cage reconstruction and tissue biopsy. Acute neurologic deterioration of both upper and lower extremities (ASIA A grade) was found 4 h after the operation upon extubation. Emergent imaging revealed no extrinsic causes. Methylprednisolone was initiated with rehabilitation therapies; her neurological status improved dramatically with complete neurological recovery at 1-year follow-up.
Conclusion: New-onset neurologic deficit is always an unexpected complication. Early identification and correct treatments can avert incomplete spinal cord from permanent damage. Our experience in dealing with this patient and following up the case for nearly 1 year showed a good neurological recovery.
Keywords: Cervical, corpectomy, fusion, reperfusion injury, white cord syndrome.
Anterior cervical corpectomy and fusion (ACCF) is the anterior approach technique used in the management of compressive myelopathy of the spinal cord and nerve root. The common complications are dysphagia, dural tear, hoarseness secondary to superior or recurrent laryngeal nerve injury, esophageal tear, vertebral artery injury, and graft-related problems [1]. A new neurologic deficit after spine surgery is always the biggest surgeon’s nightmare [2]. New-onset neurologic deficit is most often due to extrinsic causes, but few literature available till date say that worsening of neurology post-operatively in the absence of obvious per operative injury and with no extrinsic causes with the finding of hyperintense signal changes on T2-weighted magnetic resonance imaging (MRI), the deficit is attributable to be caused by reperfusion injury of the spinal cord called as white cord syndrome (WCS) [3]. Hereby, we report a case with immediate post-operative complication attributed as WCS after ACCF and; how we identified and managed this case and its status at 1-year follow-up.
A 64-year-old female with a previous 3-month history of axial neck pain with bilateral radiculopathy presented with sudden exaggerated intractable axial neck pain with progressive weakness in the last 3 days with ASIA C grade on presentation to the hospital. Computed tomography (CT) scan (Fig. 1) and MRI (Fig. 2) demonstrated C5 – C6 pathological lesion (lytic) with extradural compression with cord edema. 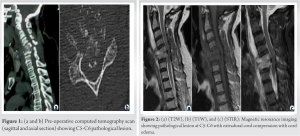
Operative procedure
Under general anesthesia, the patient underwent cervical decompression with instrumentation by an anterior approach. C5 – C6 corpectomy was carried out and removal of all debris was done until the spinal cord was adequately decompressed and then titanium mesh filled with autogenous bone graft harvested from the iliac bone was placed between C4 and C7 as anterior reconstructive procedure along with anterior cervical plating (Fig. 3); unhealthy tissues were sent for tissue diagnosis. Posterior stabilization as second-stage surgery was also part of the initial surgical plan. The patient could not be extubated on the table and was ventilated in the post-operative intensive care unit and extubated after 4 h after the surgical procedure.
Post-operative course and treatment
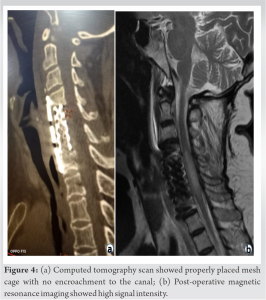
On post-extubation neurological assessment, the patient was found to be tetraplegic with loss of sensation to pain, temperature, touch, and proprioception (ASIA A grade) below the C5 level. Urgent imaging was carried out, post-operative CT scan showed the good placement of the implant with preserved alignment and no hardware malposition to cause any compression or any residual bony debris (Fig. 4a) and post-operative MRI images demonstrated worsening of cord edema extending from C4 to C7 (Fig. 4b). Immediately, she was started with high doses of methylprednisolone (30 mg/kg body weight over 15 min, followed by 5.4 mg/kg body weight over next 23 h) as per NASCIS III to reduce the edema along with rehabilitation therapies (initially at the hospital, then at home by a home-deployed physiotherapist, twice daily for next 6 months). After initiation of methylprednisolone and rehabilitation therapies, her motor and sensory status of both lower extremities recovered greatly, and achieved preoperative status on 7th post-operative day (ASIA C grade). The patient was discharged from the hospital on the 14th post-operative day with a daily rehabilitation therapies regime along with antitubercular medications; she still had bladder bowel dysfunction on discharge. Her tissue diagnosis was confirmatory with tubercular infection. The patient was regularly followed up at the interval of 1 month initially and at about 2-month follow-up, her bladder bowel dysfunction was restored to normality with improved neurology to ASIA D grade. Antitubercular medication was stopped at 9th months after assessment of healing clinically and radiologically along with blood biochemistry. She had a dramatic improvement in her neurology and had normal neurology at 1 year of follow-up (ASIA E grade).
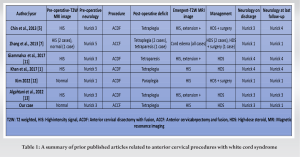
According to prior published literature, the incidence of spinal cord reperfusion injury is 0.4–18% in thoracic and abdominal aortic surgeries; and 2.87–3.89% in all spinal surgery [4]. Anterior cervical spine surgeries are common procedures with good clinical outcomes, but, sometimes, they may be associated with some serious dreaded complications such as neurologic deficit mostly caused by iatrogenic injury, followed by other possible extrinsic factors such as epidural hematoma, cerebrospinal fluid collection, hardware malposition, residual compression, systemic hypotension, and cerebrovascular insufficiency [1]. Few literatures available till date advocate that reperfusion injury (an extremely rare condition) may be associated with worsening of neurological status. A reperfusion injury in the spinal cord (also known as WCS) was first described by Chin et al. in 2013 as neurologic deterioration with concurrent hyperintense signal change on T2-weighted MRI with no obvious perioperative complications [5].
Pathophysiology
Spinal cord reperfusion injury is due to oxygen-derived free radical damage. Severity of reperfusion injury strictly depends on tissue ischemia time, extent of tissue involvement, and the affected tissue’s oxygen requirements. The spinal cord nerve is extremely sensitive to ischemia and hypoxia. Reperfusion injury is multifactorial and multichannel. This leads to ca++-mediated mitochondria-dependent apoptosis, tumor necrosis factor-alpha production, and specific phospholipid signaling cascades disrupting the blood–brain barrier or the blood–spinal cord barrier resulting in neuronal injury in human and animal models. All these changes lead to disturbance in the ionic environment and transport systems of cells leading to worsened reperfusion injury upon decompression of a previously ischemic spinal cord [5, 6, 7]. In our case, sudden decompression of the spinal cord following the removal of all debris was likely the source of acute cord injury in chronically ischemic tissue. The etiology, therefore, is attributed to rapid cord expansion with acutely increased blood supply to the affected area. These sudden changes likely resulted in disruption in the blood–brain barrier and the blood–spinal cord barrier, resulting in a reperfusion injury. Ondrejicková et al. demonstrated ischemic reperfusion injury in a rabbit model by blocking the abdominal aorta for 25 min, then allowing blood flow reperfusion for 60–120 min. They found that injury occurred only in the ischemic phase, whereas in the reperfusion phase, a tissue repair process started in the spinal cord. If reperfusion was extended to 120 min, the metabolic disorder caused by ischemia was significantly less severe [7, 8]. Torg et al. in their study, they stated that transient paralysis after anterior cervical spinal surgery may be related to spinal cord edema and the limited decompression space inherent to anterior cervical decompression procedures, rather than the ischemia-reperfusion injury [9]. Since the last decade, the perception and concept behind the worsening of neurology after spinal surgery in the absence of extrinsic causes have been changed. WCS is a rare complication with only few reported cases in the search literature and published literature from 2013 to 2022 described WCS (Table 1) as neurologic deterioration with concurrent hyperintense signal change on T2-weighted MRI in the absence of obvious perioperative complications. T2 hyperintensity is a non-specific finding but indicates an increase in water content in the area, as seen with acute ischemia or demyelination [10]. Chin et al. reported a case of WCS with pre-operative high signal intensity on T2W MRI; post-operatively, the patient developed tetraplegia with extension in high signal intensity on emergent-T2W MRI, treated with high-dose steroid along with surgical intervention [5]. Zhang et al. reported three cases of reperfusion injury with pre-operative high signal intensity in two cases and normal finding in one case on T2W MRI; post-operatively, two patients developed complete paralysis and one patient developed incomplete paralysis with cord edema finding in all cases on emergent-T2W MRI; two patients were treated with high-dose steroid only and one patient needed surgical intervention leading to improvement in neurological status in all three cases [7]. et al. reported a case with pre-operative high signal intensity on T2W MRI; post-operatively, the patient developed tetraparesis with extension in high signal intensity on emergent-T2W MRI, treated with high-dose steroid only [11]. Khan et al. reported a case with pre-operative high signal intensity on T2W MRI; post-operatively, the patient developed tetraplegia with high signal intensity on emergent-T2W MRI, treated with high-dose steroid only leading to improvement in neurological status [1]. Kim reported a case with pre-operative normal findings on T2W MRI; post-operatively, the patient developed paraplegia with high signal intensity on emergent-T2W MRI, treated with high-dose steroids along with surgical intervention [12]. Algahtani et al. reported a case with pre-operative high signal intensity on T2W MRI; post-operatively, the patient developed tetraplegia with extension in high signal intensity on emergent T2W MRI, treated with high-dose steroid only [13]. In our case, the patient had pre-operative normal findings on T2W MRI; post-operatively, the patient developed tetraplegia with high signal intensity on emergent T2W MRI, treated with high-dose steroid only leading to improvement in neurological status. Reperfusion injury-induced neurologic deficit often resolves miraculously within a few hours of timely diagnosis and treatment. Imaging modalities such as MRI often fail to identify reperfusion injury [7]. Timely intervention can quickly restore spinal function completely or partially [4, 14]. In our case, MRI images demonstrated worsening of cord edema (Fig. 4b). Immediately, the patient was started with high-dose methylprednisolone as per NASCIS III along with rehabilitation therapies. The patient showed a dramatic gradual improvement of neurological status.
New-onset neurologic deficit is always an unexpected complication. Reperfusion Injury occurs in chronically ischemic tissue and early identification and correct treatments can avert permanent damage. Proper prior counseling of the possibility of such unwanted harmful complications such as WCS before surgery should be made. Our experience in dealing with this patient with high-dose methylprednisolone and following up the case for nearly 1 year showed a good neurological recovery requiring no revision surgery.
Early diagnosis and prompt intervention can achieve full neurological recovery.
References
- 1.Khan MF, Jooma R, Hashmi FA, Raghib MF. Delayed spinal cord infarction following anterior cervical surgical decompression. BMJ Case Rep 2017;2017:r-219863. [Google Scholar]
- 2.Cramer DE, Maher PC, Pettigrew DB, Kuntz C 4th. Major neurologic deficit immediately after adult spinal surgery: Incidence and etiology over 10 years at a single training institution. J Spinal Disord Tech 2009;22:565-70. [Google Scholar]
- 3.Shan LQ, Ma S, Qiu XC, Zhou Y, Zhang Y, Zheng LH, et al. Hydroxysafflor yellow a protects spinal cords from ischemia/reperfusion injury in rabbits. BMC Neurosci 2010;11:98. [Google Scholar]
- 4.Jiang X, Shi E, Nakajima Y, Sato S. Postconditioning, a series of brief interruptions of early reperfusion, prevents neurologic injury after spinal cord ischemia. Ann Surg 2006;244:148-53. [Google Scholar]
- 5.Chin KR, Seale J, Cumming V. “White cord syndrome” of acute tetraplegia after anterior cervical decompression and fusion for chronic spinal cord compression: A case report. Case Rep Orthop 2013;2013:697918. [Google Scholar]
- 6.Pan W, Banks WA, Kastin AJ. Blood-brain barrier permeability to ebiratide and TNF in acute spinal cord injury. Exp Neurol 1997;146:367-73. [Google Scholar]
- 7.Zhang JD, Xia Q, Ji N, Liu YC, Han Y, Ning SL. Transient paralysis shortly after anterior cervical corpectomy and fusion. Orthop Surg 2013;5:23-8. [Google Scholar]
- 8.Ondrejicková O, Stolc S, Ziegelhöffer A, Horáková L, Styk J, Dzurba A, et al. Postischemic reperfusion of the spinal cord: Prolonged reperfusion alleviates the metabolic alterations induced by 25 min ischemia in the cervical and thoracolumbal segments. Gen Physiol Biophys 2000;19:415-26. [Google Scholar]
- 9.Torg JS, Corcoran TA, Thibault LE, Pavlov H, Sennett BJ, Naranja RJ Jr., et al. Cervical cord neurapraxia: Classification, pathomechanics, morbidity, and management guidelines. J Neurosurg 1997;87:843-50. [Google Scholar]
- 10.Sasamori T, Hida K, Yano S, Takeshi A, Iwasaki Y. Spinal cord swelling with abnormal gadolinium-enhancement mimicking intramedullary tumors in cervical spondylosis patients: Three case reports and review of the literature. Asian J Neurosurg 2010;5:1-9. [Google Scholar]
- 11.Giammalva GR, Maugeri R, Graziano F, Gulì C, Giugno A, Basile L, Iacopino DG. White cord syndrome after non-contiguous double-level anterior cervical decompression and fusion (ACDF): a “no reflow phenomenon”?. Interdisciplinary Neurosurgery. 2017 Mar 1;7:47-9. [Google Scholar]
- 12.Kim Y. White cord syndrome : A reperfusion injury following spinal decompression surgery. 2022;18:380-6. [Google Scholar]
- 13.Algahtani AY, Bamsallm M, Alghamdi KT, Alzahrani M, Ahmed J. Cervical spinal cord ischemic reperfusion injury: A comprehensive narrative review of the literature and case presentation. Cureus 2022;14:e28715. [Google Scholar]
- 14.Back MR, Bandyk M, Bradner M, Cuthbertson D, Johnson BL, Shames ML, et al. Critical analysis of outcome determinants affecting repair of intact aneurysms involving the visceral aorta. Ann Vasc Surg 2005;19:648-56. [Google Scholar]