This case highlights that all medical history, especially those related to the abdomen, can be important in hip prosthesis surgery.
Dr. David Popescu, Department of Orthopaedic Surgery, Cliniques Universitaires de Saint Luc, Avenue Hippocrate 10 - 1200 Woluwé Saint-Lambert, Belgium. E-mail: david.popescu@student.uclouvain.be
Introduction: Prosthetic joint infection (PJI) is a relatively infrequent occurrence; however, it always poses a significant risk to the patient’s functional outcome. The origin of PJI is often a topic of debate. In this case, we present a PJI that resulted from a digestive fistula passing through an iliopsoas abscess, which can be regarded as an unusual consequence of rare conditions.
Case Report: A 72-year-old man was brought to the emergency department with pain in his right hip and a functional impairment of his right lower limb. This had been ongoing for 3 weeks and he had no history of trauma or fever. An initial X-ray was negative for fracture, but a computed tomography scan showed a large collection of liquid and gas in his psoas major muscle extending to the right prosthetic hip. The origin of the collection was from the digestive tract. It was caused by his fistula that brought the intestinal lumen in contact with the patient’s prosthetic hip through the psoas major muscle and caused a PJI.
Conclusion: The interconnectivity of various medical disciplines is exemplified by this case, where a digestive fistula resulted in an infection of a prosthetic hip. It is essential for both the orthopedic and general surgeons to recognize that a digestive fistula can pose a threat to a prosthetic hip.
Keywords: Prosthetic hip infection, psoas abscess, fistula, digestive tract.
Prosthetic joint infection (PJI) is a rare but dreaded complication for both patients and surgeons. The current incidence of PJI in primary joint replacements is approximately 1–2%, while it increases to 4% in revision surgeries [1].
PJIs are caused by a combination of multiple risk factors, including:
- Host factors such as age, lifestyle, and medical conditions
- Pre-operative factors such as previous local surgery, active or previous infections, and septicemia.
- Surgery-related factors such as the complexity and duration of the procedure
- Post-operative factors such as hematomas, seromas, and wound healing [1, 2].
The management will be influenced by the factors mentioned above, the timing and microbiology of infection, the joint and implant condition, the soft-tissue quality, etc. PJI treatment involves long-term antibiotics and surgery. According to the variables discussed earlier, there is a spectrum from the less invasive to the most invasive approach. To achieve a good, curative or palliative, and treatment, it is essential to know the underlying cause. There are three sources known to provide PJIs. The most frequent reason is a hematogenous spread. The second cause can be direct contact of bacteria with the implant, such as per operative contamination. Moreover, less often, an infection may emerge through local dissemination from a nearby septic focus, for example, abscess [1]. We present the case of an iliopsoas abscess (IPA), caused by a fistula with the digestive tract, that led to a prosthetic hip joint infection. An IPA is a rare entity. Its incidence has yet to be assessed, but in 1980, a cohort study performed in the UK showed a rate of 0.4/100,000 population years [3]. In 1992, Gruenwald et al. estimated IPA frequency at 12 cases per year worldwide [4]. In recent study conducted in Myagi (Japan), in 2012, the estimated prevalence of IPA was 1.21/100,000 population years. According to the authors, IPA is probably underestimated given that the symptoms are atypical and not well-known [5]. IPA can be separated into two categories [4, 6].
Primary abscesses (30%)
It caused by hematogenous or lymphatic spread from a distant infected site or by a local traumatism with the development of intramuscular hematoma predisposing to abscess development.
Secondary abscesses
Due to their anatomical location, iliopsoas muscles can be infected by various contiguous spreads. Secondary IPA is most often linked to intra-abdominal inflammatory processes, which, in 60% of cases, is the consequence of Crohn’s disease. Other known origins of secondary IPA include appendicitis, colitis, diverticulitis, digestive neoplasia, urinary tract infection, or surgery. In 10% of cases, spondylodiscitis and vertebral osteomyelitis are the origin of IPA. However, it is necessary to differentiate a psoas abscess infecting a prosthetic hip and a psoas abscess secondary to a prosthetic hip infection. In the latter case, the infection spreads from distal to proximal, and the origin is the infection of the prosthesis. Psoas abscess resulting from an infected PJI complicates on average 13% of patients with an infected prosthesis but is of lesser consequence. In this case, we follow the algorithm of PJI by adding drainage of the psoas major [7, 8].
A 72-year-old man was admitted to the emergency department with functional impairment of the right lower limb and isolated right hip pain, evolving for 3 weeks. He had no history of trauma or fever. The patient had undergone a right uncemented total hip arthroplasty (DePuy Synthes, MATHYS), at our institution 7 years earlier. The patient’s medical history was notable for chronic lymphocytic leukemia, unbalanced type 2 diabetes, heart failure disease, hypertension, gout, obesity, and chronic alcoholism. In addition, he had undergone many vascular surgeries, such as bilateral femoral popliteal bypass surgery, multiple percutaneous angioplasties/stenting, and toe amputations. 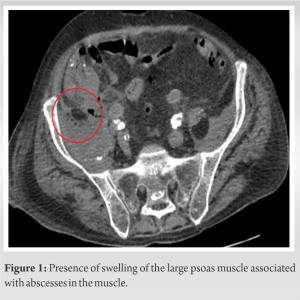
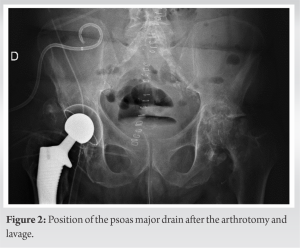
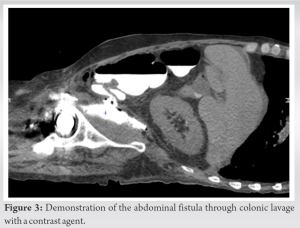
The psoas abscess was originally formed by a digestive fistula initiated at the anastomosis of the prior surgery. This is a surgical complication that has caused a fistula between the digestive tract and the psoas draining into the neoarticular cavity, resulting in contamination of the implant, which makes it unique. To the best of our knowledge, this type of case has not yet been described in the literature. Indeed, it combines the occurrence of two rare entities, a digestive fistula complicated by a psoas abscess and drainage of this abscess into the hip, with most abscesses remaining confined to the pelvis [5]. The management of an acute hip prosthetic infection is different from a chronic PJI. The type of surgery required will depend on the severity of the infection and the extent of the damage to the prosthesis. In some cases, the infected prosthesis may need to be removed and replaced with a new one, while, in others, the prosthesis may be able to be salvaged through debridement and irrigation of the infected tissue. In our case, the patient presented with sudden functional impairment of the lower limb, leading us to suspect that the abscess present in his psoas had spontaneously drained into his prosthetic hip. As <48 h had passed before he was treated, it was decided to leave the implant in place but performs a prosthetic lavage and administer antibiotics to the patient [9, 10]. A similar clinical presentation had previously been described with misaligned screws allowing passage of germs from the acetabulum to the pelvis. The patient presented with acute functional impotence of the lower limb, for which implant removal was necessary as three Muller ring fixation screws penetrated the pelvis [5]. The patient had a good outcome with a Girdlestone operation (simple resection of the prosthesis) and did not experience a septic relapse at 1 year and was able to walk with a 5 cm sole shoe. Psoas abscesses have atypical clinical presentations that can easily go unnoticed. It is therefore important in the presence of hip pain especially if associated with a febrile syndrome to perform a scan to highlight this entity. To diagnose an IPA, it is crucial to gather detailed information about the patient’s history and conduct a thorough examination [11]. A clear understanding of the symptoms, which may start off mild and gradually worsen, and any other factors that may be contributing to the condition, is crucial [6]. The abscess may be a sign of a new Crohn’s disease diagnosis or a result of a weakened immune system due to conditions such as HIV. Despite the deep location of the affected muscles, tenderness in the abdomen, discomfort during hip movements, and, in rare cases, a painless mass in the groin area, can be detected through examination [1].
This case report highlights a unique complication resulting from a psoas abscess caused by a digestive fistula and leading to contamination of a prosthetic hip. The patient’s comorbidities and poor vascular condition required a tailored surgical approach, and a good outcome was achieved with careful management. This case underscores the importance of considering rare and atypical conditions when assessing patients with hip pain and a febrile syndrome, and of conducting a thorough examination to detect signs of an IPA. Further, clinical research is needed to better understand the incidence and optimal management of this rare condition.
IPA are more likely to form spontaneously and treated with oral antibiotics and percutaneous drainage. However, it is important to exclude a digestive origin in which case the treatment is surgery. Moreover, IPA is a complicated diagnosis to make because symptoms tend to be discreet and the frequency of the pathology is rare.
References
- 1.Izakovicova P, Borens O, Trampuz A. Periprosthetic joint infection: Current concepts and outlook. EFORT Open Rev 2019;4:482-94. [Google Scholar]
- 2.Charalampopoulos A, Macheras A, Charalabopoulos A, Fotiadis C, Charalabopoulos K. Iliopsoas abscesses: Diagnostic, aetiologic and therapeutic approach in five patients with a literature review. Scand J Gastroenterol 2009;44:594-9. [Google Scholar]
- 3.Shields D, Robinson P, Crowley TP. Iliopsoas abscess--a review and update on the literature. Int J Surg 2012;10:466-9. [Google Scholar]
- 4.Gruenwald I, Abrahamson J, Cohen O. Psoas abscess: Case report and review of the literature. J Urol 1992;147:1624-6. [Google Scholar]
- 5.Buttaro M, Valle AG, Piccaluga F. Psoas abscess associated with infected total hip arthroplasty. J Arthroplasty 2002;17:230-4. [Google Scholar]
- 6.Dhinsa BS, Abdul-Jabar HB, Rajkumar S, Kochhar T. A rare case of primary psoas abscess causing hip pain in a patient with hip replacement. Acta Orthop Traumatol Turc 2014;48:598-601. [Google Scholar]
- 7.Van den Berge M, de Marie S, Kuipers T, Jansz AR, Bravenboer B. Psoas abscess: Report of a series and review of the literature. Neth J Med 2005;63:413-6. [Google Scholar]
- 8.Dauchy FA, Dupon M, Dutronc H, de Barbeyrac B, Lawson-Ayayi S, Dubuisson V, et al. Association between psoas abscess and prosthetic hip infection: A case-control study. Acta Orthop 2009;80:198-200. [Google Scholar]
- 9.Li C, Renz N, Trampuz A. Management of periprosthetic joint infection. Hip Pelvis 2018;30:138-46. [Google Scholar]
- 10.Giulieri SG, Graber P, Ochsner PE, Zimmerli W. Management of infection associated with total hip arthroplasty according to a treatment algorithm. Infection 2004;32:222-8. Erratum in: Infection 2004;32:309. [Google Scholar]
- 11.Ennaifer R, Ouakaa-Kchaou A, Belhadj N, Elloumi H, Gargouri D, Kochlef A, et al. Psoas abscess as the initial manifestation of Crohn’s disease. Report of 3 cases. Tunis Med 2009;87:340-3. [Google Scholar]