Denosumab has the potential of occurring periprosthetic AFFs like bisphosphonate.
Dr. Satomi Abe, Eniwa Hospital, 2-1-1 Koganechuou Eniwa 061-1449, Japan. E-mail: ichi0001@maple.ocn.ne.jp
Introduction: Denosumab is generally used for 5–10 years to treat postmenopausal osteoporosis. Although atypical fractures caused by bisphosphonate use are well known, reports of denosumab-associated femur fractures are rare.
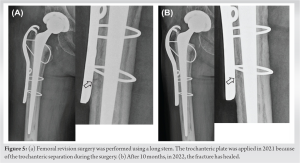
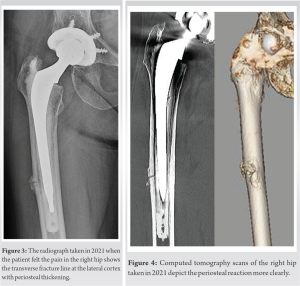
Case Report: Herein, a 75-year-old woman suffered an atypical periprosthetic femoral fracture 31 months after receiving denosumab. The fracture occurred transverse to the stem tip with lateral cortical thickening. The patient underwent revision surgery for conversion to a longer cemented stem. The fracture site healed 10 months after revision surgery.
Conclusion: As far as we know, there have been no reports of a case on periprosthetic atypical femur fracture associated with denosumab. Our study shows the potential of periprosthetic atypical femoral fractures in patients using denosumab for a long time.
Keywords: Atypical fracture, periprosthetic femoral fracture, total hip arthroplasty, denosumab, osteoporosis.
Total hip arthroplasty (THA) is widely used in patients with advanced osteoarthritic hips. THA shows excellent results and has been recognized as the surgery of the century [1]. THA has been performed for more than 60 years, and its incidence is increasing worldwide. As patients receiving THA grow older, periprosthetic femur fractures (PFFs) occur. In recent years, orthopedic surgeons have had to handle atypical femoral fractures (AFF) that the American Society for Bone and Mineral Research Task Force defined in 2010 [2]. Among PFFs, some show radiographic features consistent with AFFs, which are strongly associated to administer bisphosphonate for a long time [3, 4]. Bisphosphonates make osteoporosis patients reduce the fracture risk because of the beneficial effects of the drug. However, since bisphosphonates reduce bone remodeling, they may lead to fatigue fractures. As denosumab is a potent bone antiresorptive agent, there is a possibility of fatigue fracture following denosumab use.
A 75-year-old woman received denosumab for the 1st time in October 2018 for severe postmenopausal osteoporosis. She had no history of other medication use for osteoporosis. Osteoporosis was diagnosed both clinically and radiographically (Fig. 1). She had no nutritional deficiencies or family history of osteoporosis. She had undergone a hybrid THA because of developmental dysplasia of the right hip in 2006. At our hospital, she received denosumab 60 mg per dose, for a total of six doses. Meanwhile, colon cancer was detected in August 2019, and she underwent endoscopic surgery in September 2019. She also underwent positron emission tomography, which showed no metastasis, after which she underwent regular follow-ups without any anticancer drugs. No regular medications were prescribed for other diseases. She had been generally healthy otherwise. In May 2021, she experienced slight weight-bearing pain in the right hip with no antecedent trauma.
Due to the good long-term prognosis of THA, the incidence of PFF is rising [5], and currently, PFF is the second most common reason for revision surgery after THA [6]. With aging, it is necessary for some patients to receive medication for osteoporosis. Although antiresorptive agents, like bisphosphonate or denosumab, are the most powerful remedy for decreasing fractures in patients with osteoporosis, they reduce bone remodeling. In addition, they might lead to suppress bone turnover severely and insufficiency femoral fractures over time [7, 8]. In 2014, by the American Society for Bone and Mineral Research Task Force, the diagnosis criterion of AFF was revised as a minimum of four of the following five major features [9]: (1) Fractures associated with minor or no trauma; (2) minor or nocomminuted fractures; (3) transverse fracture line originating from the lateral cortex; (4) complete fractures extending through both cortices and possibly associated with a medial spike, while incomplete fractures involving only the lateral cortex; and (5) regional periosteal or endosteal thickening of the lateral cortex on the fracture site. The present case demonstrated a Vancouver type-B1 fracture [10] around the cemented stem. The current AFF definition excludes PPFs, however, our case fulfilled the five criteria described above. We support the study by Mondanelli et al. that claims that periprosthetic AFF exists [3]. AFF in patients using denosumab has been reported to be lower than that in patients using bisphosphonate [10, 11, 12]. Dupaix et al. [13] presented the first case of denosumab-associated peri-implant AFF. The patient in that study was treated for a subtrochanteric femur fracture with reamed intramedullary nail placement. After receiving denosumab for more than 5 years, she sustained a fracture around the locking screw placed previously. However, as far as we know, there have been no reports of denosumab-associated periprosthetic AFF. Although denosumab treatment for up to a decade showed a low fracture incidence in patients with osteoporosis and continued increases in bone mineral density with fewer adverse events rate [14], periprosthetic AFFs can occur in patients using denosumab over long periods. This study is limited by the fact that only one case is described; this is because periprosthetic AFFs do not commonly occur. However, our case demonstrates that periprosthetic AFFs may occur over time in patients undergoing denosumab treatment.
Herein, we describe a case of denosumab-associated periprosthetic AFF. Denosumab decreases fractures in osteoporotic patients by suppressing bone resorption, which reduces bone remodeling and may lead to fatigue fractures. However, it should be borne in mind that denosumab-associated periprosthetic AFFs can occur due to the high survival rate after THA and the need for osteoporotic treatments accompanying aging.
Denosumab is a good drug that has fewer adverse events and increases bone mass, though, that also has the potential of occurring periprosthetic AFFs like bisphosphonate. We should keep in mind this phenomenon under the high survival rate of THA and gaining need for osteoporotic treatment attendant on aging.
References
- 1.Learmonth ID, Young C, Rorabeck C. The operation of the century: Total hip replacement. Lancet 2007;370:1508-19. [Google Scholar]
- 2.Shane E, Burr D, Ebeling PR, Abrahamsen B, Adler RA, Brown TD, et al. Atypical subtrochanteric and diaphyseal femoral fractures: Report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2010;25:2267-94. [Google Scholar]
- 3.Mondanelli N, Facchini A, Troiano E, Muratori F, Bottai V, Giannotti S. Periprosthetic atypical femoral fractures exist: A retrospective study at a single institution. Prevalence on 115 periprosthetic femoral fractures around a primary hip stem. J Arthroplasty 2021;36:2189-96. [Google Scholar]
- 4.Schilcher J, Michaëlsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med 2011;364:1728-37. [Google Scholar]
- 5.Pagani NR, Varady NH, Chen AF, Rajaee SS, Kavolus JJ. Nationwide analysis of lower extremity periprosthetic fractures. J Arthroplasty 2021;36:317-24. [Google Scholar]
- 6.Gausden EB, Beiene ZA, Blevins JL, Christ AB, Chalmers BP, Helfet DL, et al. Periprosthetic femur fractures after total hip arthroplasty: Does the mode of failure correlate with classification? J Arthroplasty 2021;36:2597-602. [Google Scholar]
- 7.Kuroda T, Shiraki M, Shiraki Y, Tanaka S. The importance of absolute bone mineral density in the assessment of antiresorptive agents used for the prevention of osteoporotic fractures. J Clin Densitom 2012;15:392-8. [Google Scholar]
- 8.Sayed-Noor AS, Sjödén GO. Case reports: Two femoral insufficiency fractures after long-term alendronate therapy. Clin Orthop Relat Res 2009;467:1921-6. [Google Scholar]
- 9.Shane E, Burr D, Abrahamsen B, Adler RA, Brown TD, Cheung AM, et al. Atypical subtrochanteric and diaphyseal femoral fractures: Second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2014;29:1-23. [Google Scholar]
- 10.Schilcher J, Aspenberg P. Atypical fracture of the femur in a patient using denosumab--a case report. Acta Orthop 2014;85:6-7. [Google Scholar]
- 11.Drampalos E, Skarpas G, Barbounakis N, Michos I. Atypical femoral fractures bilaterally in a patient receiving denosumab. Acta Orthop 2014;85:3-5. [Google Scholar]
- 12.Thompson RN, Armstrong CL, Heyburn G. Bilateral atypical femoral fractures in a patient prescribed denosumab-a case report. Bone 2014;61:44-7. [Google Scholar]
- 13.Dupaix JP, Opanova MI, Lee LS, Christensen K. Denosumab-associated peri-implant atypical femur fracture: A case report. Hawaii J Health Soc Welf 2019;78:47-51. [Google Scholar]
- 14.Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: Results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 2017;5:513-23. [Google Scholar]