The efficacy and safety of intravenous alone with combined intravenous and intraarticular tranexamic acid.
Dr. S Noordeen, Department of Orthopedics, Srinivasan Medical College and Hospital, Trichy, Tamil Nadu. India. E-mail: drnoordeens@gmail.com
Introduction: Tranexamic acid (TXA) is an antifibrinolytic agent that reduces substantial blood loss in total knee arthroplasty (TKA) surgeries without increasing the risk of thromboembolic complications. The purpose of our study was to assess the effectiveness and safety of the combined use of intravenous and topical TXA in uncomplicated primary TKA without complications.
Materials and Methods: In this prospective study, we enrolled 61 patients who underwent unilateral primary TKR and were randomly divided into two groups: Group I received intravenous (IV) TXA and Group II received both IV and intraarticular (IA) TXA. Patients assigned to Group I received IV TXA preoperatively 30 min before surgery and postoperatively at 3 and 6 h after surgery, whereas in the combined group, in addition to IV doses, topical TXA was applied as mop 2 g of TXA diluted in 30 mL of isotonic sodium chloride solution) intraarticularly for about 5 min before closing the arthrotomy. We measured total blood loss (TBL) and mean reduction in hemoglobin (Hb) levels as primary outcomes. Transfusion rates, incidence of thromboembolic events (TE), and other adverse effects as secondary outcomes. TBL and Hb drops were noted on the 3rd post-operative day. All the patients were followed up for 6 months to note the incidence of deep venous thrombosis and TE. An independent t-test was used to evaluate between-group differences. P < 0.05 is the cutoff for statistically significant differences.
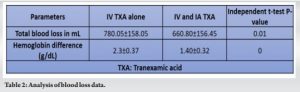
Results: The TBL in Group I was 780.05 ± 158.05 mL, compared to 660.80 ± 156.45 mL in Group II. (P < 0.001). The Hb drop was significantly lower in IV TXA group (2.3 ± 0.37) than the combined TXA group (1.40 ± 0.32). Furthermore, both groups required no transfusions. No thromboembolic complications were noted postoperatively and at 6-month follow-up.
Conclusion: TXA in total knee replacement surgery effectively decreases blood loss and significantly reduces the need for blood transfusions. Based on our study, the combined use of IV and IA TXA in total knee replacement was found to be superior in reducing blood loss and significantly reducing the need for blood transfusions in TKA.
Keywords: Total knee arthroplasty, blood loss, tranexamic acid, intraarticular route, intravenous route.
Total knee arthroplasty (TKA) is a commonly performed elective surgical procedure for patients with debilitating arthritis. The prevalence of TKA procedure is increasing, as it can reduce joint pain and improve joint function and quality of life in arthritis patients [1-3]. However, it is often associated with a significant risk of blood loss which can lead to anemia, an increase in the rate of allogenic blood transfusions, and prolonged hospital stays. Blood transfusions lead to serious complications such as transfusion reactions, surgical wound infections, and immunological reactions. Furthermore, increases the length of stay in the hospital, thereby increasing the cost of treatment [4]. A prior comprehensive study revealed that TKR stands among the top 10 procedures in the USA that often necessitate blood transfusions. Moreover, there were blood transfusion-associated serious complications, such as wound infection, an increase in hospital stay, allogeneic blood reaction, in-hospital mortality [5-7], and deep venous thromboembolism. Hence, perioperative blood management strategies focus on minimizing blood loss and the need for blood transfusions. Preventing bleeding around the knee reduces post-operative pain, hemarthroses, limb swelling, and facilitates better post-operative functional outcomes. The use of a tourniquet was a common practice in TKA surgeries to provide a bloodless field and to enhance visualization [8]. However, many studies have reported complications such as thigh pain, limb swelling, paralysis, delayed nerve recovery, and injury to soft tissue, muscles, calcified vessels, and nerves, related to tourniquet use [9-12]. Even the tourniquet use increases the risk of venous emboli and ischemic reperfusion injury [13]. Tranexamic acid (TXA) is a synthetic derivative of the amino acid lysine, known for its hemostatic properties [14, 15]. Its mechanism of action revolves around its ability to inhibit fibrinolysis, the process by which blood clots dissolves, thereby promoting clot stability and reducing bleeding. Hence, TXA has found widespread application in various medical specialties, including orthopedic surgery, trauma care, dentistry, and obstetrics [16, 17]. In orthopedic procedures such as TKA, TXA is frequently utilized to minimize intraoperative and post-operative blood loss, thereby decreasing the need for blood transfusions and associated complications. Various studies confirmed the perioperative use of TXA provides a bloodless surgical field, reduces surgical drains, and thus prevents the need for allogeneic blood transfusion, and surgical drain [18-20]. Many studies have shown the different routes of administration of TXA including intravenous (IV), oral, and topical. IV administration is commonly employed for systemic delivery, providing widespread antifibrinolytic effects throughout the body. Topical application, on the other hand, involves direct placement of TXA at the surgical site, enabling targeted action and minimizing systemic exposure. While systemic administration of TXA has shown promising results in TKA, concerns regarding potential thromboembolic events (TE) have prompted interest in exploring alternative administration routes and dosages. Combining systemic and topical administration of TXA presents a novel approach that aims to increase its hemostatic effects while minimizing systemic exposure and associated risks. Hence, this prospective study is aimed to analyze the safety and efficacy of TXA in patients undergoing TKA.
This prospective, open-label, comparative clinical study was designed and conducted in the departments of orthopedics outpatient department (OPD). This is a single-blinded study where the participants are blinded. All patients attending orthopedic OPD with primary osteoarthritis of knee joints were assessed in the OPD and those willing for surgery were scheduled for elective surgery. All the consecutive unilateral TKA were randomly assigned to two groups in 1:1 ratio by simple randomization.
Inclusion criteria
All adult patients aged between 50 and 80 years with Grade III and IV (Kellgren–Lawrence) osteoarthritis who were undergoing elective unilateral primary TKA were included in the study.
Exclusion criteria
The patients with,
- Revision knee
- Bilateral TKA
- Post-traumatic arthritis
- Inflammatory arthritis
- Any additional procedure other than TKA
- A history of venous thromboembolic disease
- Known allergy to TXA
- Patients on anticoagulants or antiplatelet drugs,
- Any underlying diseases such as cirrhosis, chronic renal failure, hemostasis, and
- Pre-operative hemoglobin (Hb) <10 g/dL.
The study was conducted after the clearance from the Institutional Ethics Committee. This study was conducted in accordance with the principles of good clinical practice and the declaration of Helsinki for biomedical research involving human subjects. All the patients had provided written informed consent. Routine laboratory investigations, chest X-rays, echocardiograms, and electrocardiograms (ECGs) were done. Before surgery, cardiologists and anesthetists evaluated the patient’s fitness status for surgery. Two units of packed cells were arranged in all cases before surgery. Based on the study by Pinsornsak and Chumchuen [21], the sample size was calculated.
TXA administration
All the patients in both groups received a calculated dose of IV TXA (preoperatively 15 mg/kg 5–10 min before skin incision and 10 mg/kg postoperatively at 3 and 6 h after the first dose). Patients in Group II received both IV TXA and intraarticular (IA) TXA, along with IV TXA, a mop soaked in TXA solution (prepared by mixing 2g TXA in 100 mL of isotonic sodium chloride solution) was applied intraarticularly for 5 min before the closure of the incision.
Surgical procedure and perioperative management
All surgical procedures were done by the same surgeon through a minimally invasive sub-vastus approach under regional anesthesia. No tourniquet and no drain were applied in our study. All patients received antibiotic prophylaxis with IV cefuroxime 1.5 g preoperatively followed by a post-operative oral dose of 750 mg 8 hourly for the next 2 days. To reduce post-operative pain control and for early recovery, continuous femoral nerve block was given for 24 h. Then IV diclofenac sodium75 mg infusion was given in case of normal serum creatinine level, otherwise, injection of paracetamol 1000 mg infusion was given 8 hourly in raised creatinine level. As deep venous thrombosis (DVT) prophylaxis, an injection of enoxaparin was given postoperatively. Followed by factor Xa inhibitor rivaroxaban 10 mg for 2 weeks along with tablet aspirin 75 mg for 4 weeks was given. Early mobilization and strengthening exercises were encouraged. For both lower limbs, below-knee stockings were advised for 6 weeks.
Outcome assessment
The primary outcomes were a reduction in total blood loss (TBL) and post-operative Hb drop. Hb was recorded on 3rd post-operative day. The post-operative Hb drop was determined from the difference between the pre-operative Hb and the post-operative Hb level (3rd post-operative day before the patient was routinely discharged from the hospital). The TBL was calculated using Hb balance method based on patient blood volume (PBV), Hb loss, and the formula described in previous studies [14, 15]. The secondary outcomes were the blood transfusion rate and the incidences of symptomatic DVT and TE. Routine ultrasonography for screening of DVT and TEs was not performed in all patients; instead, it was advised only in patients having symptoms of DVT such as pain, swelling, or presence of Hooman’s sign. All patients were followed up for 6 months to monitor the incidence of DVT and TE. The PBV can be calculated using the formula of Nadler formula [22]. HB loss was calculated from the difference between the pre-operative Hb and the post-operative Hb level on the 3rd post-operative day before the patient was discharged. Peri-operative blood loss was calculated by the Hb balance method indirectly.
Statistical analysis
The statistical analysis was performed using SPSS software 19. The continuous data with normal distribution were expressed as means±standard deviation. Independent t-test was used to evaluate between-group differences. P < 0.05 is the cutoff for statistically significant differences.
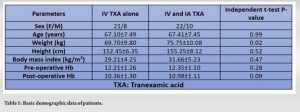
A total of 67 patients were assessed in the ortho OPD and three patients with inflammatory arthritis, two patients with bilateral TKA, and one patient with post-traumatic arthritis were excluded from the study. At the end of the study, 61 patients underwent unilateral TKA and completed the study. Their ages ranged from 50 to 80 years including 18 men and 43 women with the mean age of in Group I was 67.10 ± 7.49 and 67.41 ± 7.45 in Group II) (Table 1).
There were no significant differences in pre-operative body mass index, Hb, between the two groups (Table 1). The TBL in Group I (IV TXA alone) was 780.05 ± 158.05 mL, compared to 660.80 ± 156.45 mL in Group II (P < 0.001) (Table 2). The Hb drop was significantly lower in IV TXA group (2.3 ± 0.37) than the combined TXA group (1.40 ± 0.32) (Table 2).
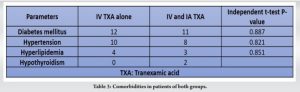
Blood transfusion was not needed in any group and no thromboembolic complications were noted postoperatively. Table 3 shows the comorbidities of the patients. The results demonstrated a significant reduction in TBL, HBL, and maximum Hb drop in the combined IV and topical TXA group compared to IV alone group. No thromboembolic complications were noted postoperatively and at 6-month follow-up. None of the patients had post-operative complications such as nerve palsies, surgical wound infections, hemarthrosis, or skin necrosis.
The present study had the following findings (1) TXA has demonstrated a significant reduction in TBL, maximum Hb drop, and transfusion rates (2) TXA reduces blood loss without an increase in thromboembolic complications. (3) Combined IV and topical TXA use has better efficacy and safety compared to IV alone group. A recent study [22] has reported the better efficacy of combined IV and topical TXA compared with topical TXA use alone. We conducted this study to evaluate the efficacy and safety of IV administration of TXA alone versus combined use of IV and IA TXA. We did not use a tourniquet in our study [9-12]. Because many studies have reported tourniquet-related adverse events, the current evidence does not support the use of tourniquets. A study has reported tourniquet-induced ischemic reperfusion injury resulting in atrophy [23]. The commonly used strategies in perioperative blood management include treating pre-operative anemia with IV iron preparations, perioperative TXA, and by restricting transfusion protocol postoperatively [24]. TXA can be administrated orally, through IV infusion, and by topical application. Literature supports TXA through any route can effectively reduce blood loss and transfusion rates in TKA [25-28]. Many literatures have reported that oral TXA as better regimen for reducing blood loss in TKA [29-31]. However, TXA is mainly administrated by IV infusion and topical application. In the present study, sub-vastus approach was employed and 15 mg/kg of TXA was administered IV, 5–10 min before skin incision, and 10 mg/kg was given postoperatively at 3- and 6- h after the first dose. For topical administration, a mop soaked in TXA solution (prepared by mixing 2g TXA in 100 mL of isotonic sodium chloride solution) was applied intraarticularly for 5 min before the closure of the incision. The dosing regimen in this study was based on previous studies [32-34] A two-dose regimen with a pre-operative dose and intraoperative dose is considered the least necessary regimen for effective reduction in drain loss and TBL [35]. A meta-analysis which included 15 randomized controlled trials reported that IV TXA significantly reduced blood loss and blood transfusion units, without increasing the risk of DVT or pulmonary embolism [30]. A study by Gomez-Barrena et al. showed that topical application of TXA reduces blood loss in TKA. This study showed that 3 g of topical TXA is as effective as IV TXA (15 mg/kg TXA before releasing tourniquet and 3 h postoperatively) [25]. Previous studies have shown that TXA has reduced drainage volume by about 50% [36-38]. About 10–15 mg/kg of TXA was given before the release of the tourniquet and in one study, the TXA was given before application of the tourniquet [39]. Venous thromboembolism (VTE) consists of pulmonary embolism (PE) and DVT, and it has been recognized as a significant issue in public health [40, 41]. The mortality rates associated with VTE after lower limb arthroplasty are minimal. Before 1980, the prevalence rate of VTE without prophylaxis was approximately 15–30%. However, after the use of contemporary preventive techniques in 2001, this rate has been decreased to 1–2% [42]. The risk of VTE is greatly increased when undergoing total hip arthroplasty and TKA. Without proper prophylaxis, the estimated prevalence rate of VTE is 60% [43]. A meta-analysis compared combined IV and topical TXA and IV TXA and topical TXA. There was reduced TBL, hidden blood loss, drainage volume, a lower transfusion rate, and a lower HB drop (P < 0.05), and was not associated with a higher risk such as wound infection and DVT (P > 0.05) [44]. TXA did not have fibrinolytic activity in veins [37-39], that explains the reason for not increasing the incidence of DVT. Even in the present study, there was no TE in the 6-month follow-up period. This study has certain limitations. The sample size was small so we were underpowered to detect uncommon events such as VTEs. Moreover, routine Doppler to rule out DVT or any TEs was not done, instead guided by symptoms and signs of DVT or TEs. Hence further study in multicenter with larger samples is needed to draw conclusions.
We conclude that TXA in total knee replacement surgery effectively decreases blood loss and significantly reduces the need for blood transfusions. Based on our study, the combined use of IV and IA TXA in total knee replacement was found to be superior in reducing blood loss in TKA.
Findings from our study underscore the positive functional outcomes achieved through surgical intervention utilizing posterior stabilization and fusion for spondylolisthesis, highlighting its potential as an effective treatment approach to enhance patient quality of life and alleviate symptoms.
References
- 1.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2014;94:267-73. [Google Scholar]
- 2.Bozic KJ, Ward L, Vail TP, Maze M, Bundled Payments for Care Improvement Initiative Orthopedic Working Group. Bundled payments in total joint arthroplasty: Targeting opportunities for quality improvement and cost reduction. Clin Orthop Relat Res 2015;473:49-56. [Google Scholar]
- 3.Chen J, Chiu YL, Chen LH, Lin SJ, Lo WH, Hsu YC. Continuous wound infusion system in TKA reduces pain and improves early knee function: A randomized controlled study. Clin Orthop Relat Res 2014;472:1502-7. [Google Scholar]
- 4.Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion 2010;50:753-65. [Google Scholar]
- 5.Kong L, Cao J, Zhang Y, Ding W, Shen Y. Risk factors for periprosthetic joint infection following primary total hip or knee arthroplasty: A meta-analysis. Int Wound J 2017;14:529-36. [Google Scholar]
- 6.Minnema B, Vearncombe M, Augustin A, Gollish J, Simor AE. Risk factors for surgical-site infection following primary total knee arthroplasty. Infect Control Hosp Epidemiol 2004;25:477-80. [Google Scholar]
- 7.Poeran J, Ippolito K, Brochin R, Zubizarreta N, Mazumdar M, Galatz LM, et al. Utilization of drains and association with outcomes: A population-based study using national data on knee arthroplasties. J Am Acad Orthop Surg 2019;27:e913-9. [Google Scholar]
- 8.Jones RE. Total knee arthroplasty without the use of a tourniquet. Semin Arthroplasty 2011;22:176-8. [Google Scholar]
- 9.Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br 1995;77:250-3. [Google Scholar]
- 10.Din R, Geddes T. Skin protection beneath the tourniquet. A prospective randomized trial. ANZ J Surg 2004;74:721-2. [Google Scholar]
- 11.Tai TW, Chang CW, Lai KA, Lin CJ, Yang CY. Effects of tourniquet use on blood loss and soft-tissue damage in total knee arthroplasty: A randomized controlled trial. J Bone Joint Surg Am 2012;94:2209-15. [Google Scholar]
- 12.Alcelik I, Pollock RD, Sukeik M, Bettany-Saltikov J, Armstrong PM, Fismer P. A comparison of outcomes with and without a tourniquet in total knee arthroplasty: A systematic review and meta-analysis of randomized controlled trials. J Arthroplasty 2012;27:331-40. [Google Scholar]
- 13.Arthur JR, Spangehl MJ. Tourniquet use in total knee arthroplasty. J Knee Surg 2019;32:719-29. [Google Scholar]
- 14.Orpen NM, Little C, Walker G, Crawfurd EJ. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: A prospective, randomized, controlled trial of 29 patients. Knee 2006;13:106-10. [Google Scholar]
- 15.Dhillon MS, Bali K, Prabhakar S. Tranexamic acid for control of blood loss in bilateral total knee replacement in a single stage. Indian J Orthop 2011,45:148-52. [Google Scholar]
- 16.Charoencholvanich K, Siriwattanasakul P. Tranexamic acid reduces blood loss and blood transfusion after TKA: A prospective randomized controlled trial. Clin Orthop Relat Res 2011;469:2874-80. [Google Scholar]
- 17.Bakti FU, Fitriasari N, Wahyuni IS. The use of tranexamic acid mouthwash in the gingival bleeding management in aplastic anemia patient. Int J Appl Pharm 2023;15:63-7. [Google Scholar]
- 18.Albasha A, Salman LA, Elramadi A, Abudalou A, Mustafa A, Hejleh HA, et al. Outcomes of drain versus no drain in total knee arthroplasty: A retrospective cohort study. Int Orthop 2023;47:2985-9. [Google Scholar]
- 19.Al-Zahid S, Davies AP. Closed suction drains, reinfusion drains or no drains in primary total knee replacement? Ann R Coll Surg Engl 2012;94:347-50. [Google Scholar]
- 20.Xu H, Xie J, Lei Y, Huang Q, Huang Z, Pei F. Closed suction drainage following routine primary total joint arthroplasty is associated with a higher transfusion rate and longer postoperative length of stay: A retrospective cohort study. J Orthop Surg Res 2019;14:163. [Google Scholar]
- 21.Pinsornsak P, Chumchuen S. Can a modified Robert Jones bandage after knee arthroplasty reduce blood loss? A prospective randomized controlled trial. Clin Orthop Relat Res 2013;471:1677-81. [Google Scholar]
- 22.Ueno M, Sonohata M, Fukumori N, Kawano S, Kitajima M, Mawatari M. Comparison between topical and intravenous administration of tranexamic acid in primary total hip arthroplasty. J Orthop Sci 2016;21:44-7. [Google Scholar]
- 23.Chen S, Li J, Peng H, Zhou J, Fang H, Zheng H. The influence of a half-course tourniquet strategy on peri-operative blood loss and early functional recovery in primary total knee arthroplasty. Int Orthop 2014;38:355-9. [Google Scholar]
- 24.Lu Q, Peng H, Zhou GJ, Yin D. Perioperative blood management strategies for total knee arthroplasty. Orthop Surg 2018;10:8-16. [Google Scholar]
- 25.Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Pérez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: A double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am 2014;96:1937-44. [Google Scholar]
- 26.Lin SY, Chen CH, Fu YC, Huang PJ, Chang JK, Huang HT. The efficacy of combined use of intraarticular and intravenous tranexamic acid on reducing blood loss and transfusion rate in total knee arthroplasty. J Arthroplasty 2015;30:776-80. [Google Scholar]
- 27.Pitta M, Zawadsky M, Verstraete R, Rubinstein A. Intravenous administration of tranexamic acid effectively reduces blood loss in primary total knee arthroplasty in a 610-patient consecutive case series. Transfusion 2016;56:466-71. [Google Scholar]
- 28.Serrano Mateo L, Goudarz Mehdikhani K, Cáceres L, Lee YY, Gonzalez Della Valle A. Topical tranexamic acid may improve early functional outcomes of primary total knee arthroplasty. J Arthroplasty 2016;31:1449-52. [Google Scholar]
- 29.Alipour M, Tabari M, Keramati M, Zarmehri AM, Makhmalbaf H. Effectiveness of oral Tranexamic acid administration on blood loss after knee artroplasty: A randomized clinical trial. Transfus Apher Sci 2013;49:574-7. [Google Scholar]
- 30.Irwin A, Khan SK, Jameson SS, Tate RC, Copeland C, Reed MR. Oral versus intravenous tranexamic acid in enhanced-recovery primary total hip and knee replacement: Results of 3000 procedures. Bone Joint J 2013;95-B:1556-61. [Google Scholar]
- 31.Ye W, Liu Y, Liu WF, Li XL, Shao J. The optimal regimen of oral tranexamic acid administration for primary total knee/hip replacement: A meta-analysis and narrative review of a randomized controlled trial. J Orthop Surg Res 2020;15:457. [Google Scholar]
- 32.Shah N, Gupta A, Patel D. Strategies to decrease blood loss in patients who undergo total knee replacement: A prospective study of one hundred and fifty cases. Reconstr Rev 2013;3:18-26. [Google Scholar]
- 33.Shah N, Nilesh G, Patel N. Mini-subvastus approach for total knee arthroplasty in obese patients. Indian J Orthop 2010;44:292-9. [Google Scholar]
- 34.Shah N, Khetan V, Sivanadan H. Should tranexamic acid be used for 3 days after total knee replacement? A randomized study in 250 patients. Acta Orthop Belg 2021;87:697-703. [Google Scholar]
- 35.Maniar RN, Kumar G, Singhi T, Nayak RM, Maniar PR. Most effective regimen of tranexamic acid in knee arthroplasty: A prospective randomized controlled study in 240 patients. Clin Orthop Relat Res 2012;470:2605-12. [Google Scholar]
- 36.Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: A meta-analysis. J Bone Joint Surg Am 2012;94:1153-9. [Google Scholar]
- 37.Hiippala S, Strid L, Wennerstrand M, Arvela V, Mäntylä S, Ylinen J, et al. Tranexamic acid (Cyklokapron) reduces perioperative blood loss associated with total knee arthroplasty. Br J Anaesth 1995;74:534-7. [Google Scholar]
- 38.Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: A prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg Br 1996;78:434-40. [Google Scholar]
- 39.Hiippala ST, Strid LJ, Wennerstrand MI, Arvela JV, Niemelä HM, Mäntylä SK, et al. Tranexamic acid radically reduces blood loss and transfusions associated with total knee arthroplasty. Anesth Analg 1997;84:839-44. [Google Scholar]
- 40.Jansen AJ, Andreica S, Claeys M, D’Haese J, Camu F, Jochmans K. Use of 'tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. Br J Anaesth 1999;83:596-601. [Google Scholar]
- 41.Ilinčić B, Stokić E, Stošić Z, Kojić NE, Katsiki N, Mikhailidis DP, et al. Vitamin D status and circulating biomarkers of endothelial dysfunction and inflammation in non-diabetic obese individuals: A pilot study. Arch Med Sci 2017;13:53-60. [Google Scholar]
- 42.Erem C, Kuzu UB, Deger O, Can G. Prevalence of gestational diabetes mellitus and associated risk factors in Turkish women: The Trabzon GDM Study. Arch Med Sci 2015;11:724-35. [Google Scholar]
- 43.Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012;141:S278-325. [Google Scholar]
- 44.Dimitrov BD, Bahchevanov KM, Atanassova PA, Mitkov MD, Massaldjieva RI, Chompalov KA, et al. Metabolic syndrome severity score: Range and associations with cardiovascular risk factors. Arch Med Sci Atheroscler Dis 2016;1:e90-7. [Google Scholar]