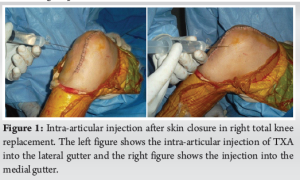
Intra-articular tranexamic acid injections can be an effective and safe method for reducing blood loss and transfusion needs in patients undergoing total knee arthroplasty.
Dr. Mainak Roy, Department of Orthopaedics, All India Institute of Medical Sciences, Bhubaneswar, Odisha, India. E-mail: mainakroy30@gmail.com
Introduction: One of the primary issues following total knee arthroplasty (TKA) is blood loss. According to the research, tourniquet time which works by inducing fibrinolysis activity is one of the primary factors contributing to blood loss. This element gives the surgeon the chance to reduce blood loss, which subsequently avoids allogenic blood transfusion and any associated risks. Both oral and intravenous administrations of tranexamic acid (TXA) are acceptable. There is disagreement over the ideal administrative mode. Because TXA is delivered locally, it has a higher chance of being both safe and effective at the target site due to the drug’s low systemic absorption.
Materials and Methods: We conducted a prospective trial involving 50 unilateral TKA procedures. In 25 TKR following closure, intra-articular TXA was administered, and in 25 TKR, 0.9% normal saline of equivalent volume was provided. Hemoglobin, hematocrit, and the number of patients needing blood transfusions and blood collection in the drain after surgery were used to gauge the effectiveness. Complications were assessed using lower leg Doppler ultrasonography to detect deep vein thrombosis (DVT).
Results: Despite the control group receiving more blood transfusions, we observed a substantial decrease in hemoglobin in the TXA group (P < 0.002) and a significant decrease in drain collection (95% CI: 203.8–406.2, P < 0.001). There were no DVT patients in.
Conclusion: TXA injected intra-articularly appears to be a safe and effective way to lessen post-TKA blood loss and the need for blood transfusions.
Keywords: Tranexamic acid injections, Total knee arthroplasty, Blood loss reduction, Deep vein thrombosis; Post-operative complications
Blood loss is a major side effect of total knee arthroplasty (TKA) that cannot be avoided but can be minimized during the perioperative phase with several strategies. Some patients may require allogeneic blood transfusions as a result of blood loss, and serious complications have been reported, despite the low prevalence [1]. Multiple studies have demonstrated that the use of tourniquets causes blood loss by affecting fibrinolytic and coagulation systems, which can be managed with pharmaceuticals. Surgeons have leveraged this to minimize blood loss [2]. Numerous studies have demonstrated that using tranexamic acid (TXA) during TKA reduces blood loss [3]. TXA is an antifibrinolytic medication that decreases blood loss by stabilizing clots, typically broken up by plasmin generated by altered fibrinolysis due to the tourniquet. It is generally accepted that only a small percentage of the intravenously injected drug reaches the target location, and it is thought that the mechanism of TXA action is inhibition of tissue fibrinolysis and stabilization of clots by TXA in the extravascular space, accumulating in tissues for up to 17 h [4]. Thus, the optimal mode of administration is debated [5]. We hypothesized that patients undergoing total knee replacement would benefit from intra-articular TXA due to its safety and efficacy.
Study design
A Prospective Randomized Placebo-Controlled Study.
A total of 50 patients undergoing elective unilateral cemented TKA were included in the study after Institutional Ethics Committee approval and informed written consent from patients. Patients under 80 years old who gave their consent and had been diagnosed with osteoarthritis (OA) of the knee in either sex were included. The following were the inclusion criteria: i) Every single initial total knee replacement case. The following were the exclusion standards:(1) Revision TKR; (2) medication allergy, known or suspected; (3) acquired or inherited hemostatic disease; (4) abnormal coagulation screening test (platelet count, prothrombin time, APT T); (5) aspirin consumption within 7 days post-surgery; (6) renal/hepatic insufficiency; (7) pregnancy; (8) history of pulmonary embolism or deep vein thrombosis (DVT); (9) history of ocular pathology or ophthalmological procedures other than corrective lenses; and (10) history of hematuria. The blocked randomization was generated by STATA 11.0 software (Department of Biostatistics, AIIMS), with a block size of four, and further concealed with sealed envelopes in the sequentially numbered container. The envelopes were sequentially opened intraoperatively after skin closure. Then, all patients were allocated to one of two groups: The TXA group or the control group. In both the TXA and control groups, the solution was prepared as a total of 20 mL volume in the same-size syringe, according to the allocation received, under sterile conditions. The solution was injected into the knee joint after completion of skin closure to prevent leakage (Fig. 1). In the TXA group, the solution contained 1000 mg of TXA in 10 mL of physiologic saline. In the control group, the solution was only 20 mL of physiologic saline. Both prepared solutions had the same appearance.
Following surgery, the need for blood transfusions was evaluated if any of the following conditions were met: (1) hematocrit of 28%; (2) drain collection exceeding 500 mL (indicating potential continuous blood loss) within the first 8–10 h; and (3) the patient experienced subjective anemia symptoms (hypotension, tachycardia, and dyspnea). Before taking any corrective action, the patient’s hematological profile was completed urgently if only the second problem (item-2) was present. The patient’s clinical and hematological status was reassessed and one unit of red blood cells (RBC) was transfused if transfusion was deemed necessary. Maintaining a hematocrit >28% or stabilizing the unbearable symptoms of anemia was the goal of blood transfusions. The number of patients transfused and the total number of units transfused in each group were used to track the need for blood transfusions. The transfusion guidelines aligned with the blood transfusion policy of our institution.
Statistical analysis
Data were analyzed by using STATA 12 (Department of Biostatistics, AIIMS). Data are represented as the mean (standard deviation [SD])/median (min & max) and frequency (%). Qualitative data (categorical data) were compared between the groups by the Chi2 test/Fischer test. Continuous data (quantitative data) following the normal distribution were compared between the groups by independent T-tests, and within changes in each group were seen by repeated-measures ANOVA (2-way ANOVA) followed by post-operative Bonferroni correction.
Observations and Results
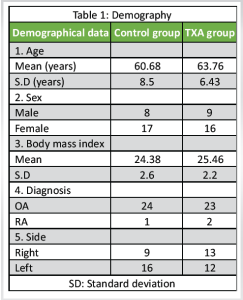
A total of 50 OA knees who presented to the Department of Orthopedics, All Institute of Medical Sciences between May 2022 and May 2023 who required surgical intervention and fulfilled the inclusion and exclusion criteria were included in the study. The patients were randomized into two groups TXA group and a control group. We included 53 knee joints with OA knee. Two patients in the control group and one patient in the TXA group were lost to follow-up and could not be contacted. Of the remaining 50 knee joints with OA, 25 OA knees in the TXA group and 25 OA knees in the control group were studied for 6 months post-surgery.
Demography: Table 1
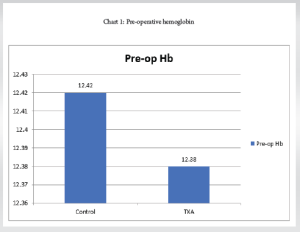
Pre-operative Hemoglobin (Table 2 and Chart 1)
The mean pre-operative Hemoglobin of 50 patients was 12.38 with an SD of 0.769.
Control group: The mean pre-operative Hemoglobin of 25 patients was 12.42 with an SD of 0.84.
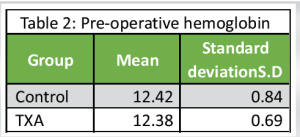
TXA group: The mean pre-operative Hemoglobin of 25 patients was 12.38 with a SD of 0.69.
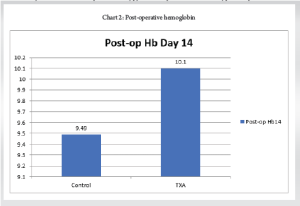
Post-operative day 14 hemoglobin
The mean post-operative Hemoglobin was 9.8 with a SD of 0.72. (Table 3 and Chart 2)
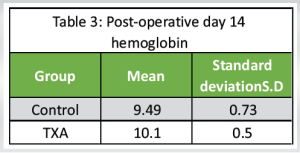
Control group: The mean Hemoglobin on a post-operative day 14 was 9.49 with an SD of 0.73.
TXA group: The mean Hemoglobin on a post-operative day 14 was 10.10 with an SD of 0.50.
Pre-operative hematocrit
The mean pre-operative Hematocrit in 50 patients was 36.38 with a SD of 1.6. (Table 4)
Control group: The mean pre-operative Hematocrit in 25 patients was 36.42 with an SD of 1.7.
TXA group: The mean pre-operative Hematocrit in 25 patients was 36.34 with an SD of 1.5.
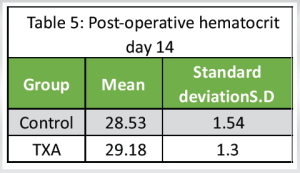
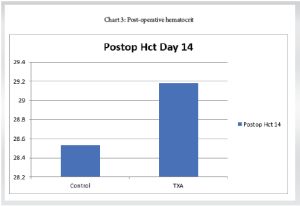
Post-operative hematocrit day 14
The mean Hematocrit on post-operative 14 was 29.18 with an SD of 1.56 (Table 5).
Control group: The mean Hematocrit on a post-operative on day 14 was 28.53 with a SD of 1.54.
TXA group: The mean Hematocrit on post-operative on day 14 was 29.18 with a SD of 1.30
(Chart 3).
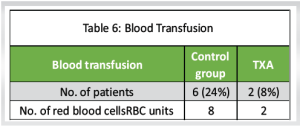
Blood transfusion (Table 6)
Control group: The number of patients who required blood transfusion was 6 with a total of 8 RBC units needed in the control group
TXA group: The number of patients who required blood transfusion was 2 with a total of 2 RBC units needed
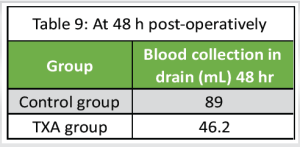
Blood collection in drain (Tables 7, 8, 9)
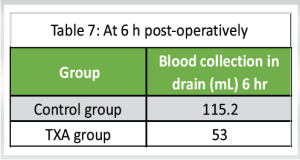
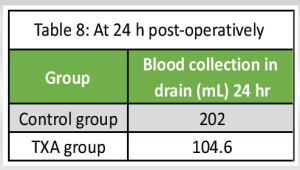
Control group: The mean blood collection in drain at 6 h, 24 h, and 48 h post-operatively was 115 ± 10.6 mL, 202 ± 8.6 mL, and 89 ± 8.7 mL, respectively, with “P” < 0.0001
TXA group: The mean blood collection in drain at 6 h, 24 h, and 48 h post-operatively was 53 ± 8.7 mL, 104.6 ±7.8 mL, and 46.2 ± 8.3 mL, respectively, with “P” < 0.001.
Doppler ultrasonography of lower limb
In our study, all patients of the control group and TXA group were found to be negative in the result of Doppler ultra-sonography of lower limbs which was done on the 7th day, 6th week, and 3rd month post-operatively (Fig. 1).
Our study, which included 50 patients, revealed a mean age of 62.22 years. Our analysis revealed a significantly lower mean age, maybe because of regional variations in the population with a higher percentage of female participants. Our study’s mean BMI values were comparable to those reported by Roy et al., Ishida et al., Seo et al., and Sa-Ngasoongsong et al., all of these studies were within the high-normal range [6-9]. In terms of the surgical side that was operated, our analysis revealed a marginally larger number of left knee joints performed. The study found that, with a P = 0.51, the mean operation length for the control group and TXA group was 60.5 ± 2.2 min and 60.9 ± 2.1 min, respectively. Comparable results to our study were found in Seo et al.’s study, which reported mean surgery durations of 54.6 min in the intra-articular injection group, 55.1 min in the IV injection group, and 55.1 min in the placebo group [8]. Factors, such as laterality, surgeon experience, and surgical complexity can affect variability in operation times. Our findings showed that, on average, the control group’s tourniquet stayed inflated for 66.2 ± 2.0 min throughout surgery, whereas the TXA group’s tourniquet remained inflated for 66.4 ± 2.1 min. A P = 0.73. Surprisingly, tourniquet time was not disclosed in any of the research projects carried out by Ishida et al., Seo et al., and Sa-Ngasoongsong et al. [7-9]. In our trial, intra-articular TXA was administered after the surgery was finished, and the tourniquet was deflated. In addition, our study showed a mean of 12.38 g/dL in 50 patients’ pre-operative hemoglobin levels, which is similar to values reported by Sa-Ngasoongsong et al. [9], Roy et al. [6], Ishida et al. [7] and Seo et al. [8] highlighting consistency in pre-operative patient profiles across studies. In this study, we evaluated the effects of TXA on a range of parameters in patients who had TKA. With a P = 0.0023, we observed a significant difference in post-operative hemoglobin on day 14 between the TXA group (mean: 10.10 ± 0.59 mg/dL) and the control group (mean: 9.49 ± 0.73 mg/dL). This is consistent with the results of Roy et al.’s investigation, which showed notable variations on day 5 following surgery [6]. Post-operative hemoglobin levels were also found to be significantly lower, according to Ishida et al. [7]. The pre-operative hematocrit values in our investigation were in line with Roy et al.’s findings [6], our study’s post-operative hematocrit on day 14 revealed substantial differences between the TXA and control groups. The TXA group showed a reduced drop in the trend of hemoglobin and hematocrit levels after surgery, with peak falls usually happening on the 7th day after surgery. Our study and others showed that the TXA group required fewer blood transfusions; however, these differences were not always statistically significant. According to results from prior trials, the TXA group had much less drainage blood loss at different times. This implies that TXA helps TKA patients require fewer transfusions and less post-operative blood loss. In all of the patients in our study, we performed Doppler ultrasonography on the lower leg after surgery on the 7th, 6th, and 3rd months. In neither group did we identify clinically asymptomatic DVT. According to the Ishida et al. study [7], neither group experienced any symptoms of VTE, nor the D-dimer assay findings showed no discernible difference between the two groups. However, clinically asymptomatic VTE was not detected using techniques, such as ultrasonography, and it remained unclear what the actual incidence ratio was. Similarly, research by Seo et al. [8] demonstrated that intra-articular injection of TXA did not result in any thromboembolic consequences, such as DVT or pulmonary embolism. Alshryda et al. [10] conducted a systematic review and meta-analysis of randomized controlled trials evaluating the effect of TXA on blood loss and transfusion in primary total knee replacement. The review used the generic evaluation tool designed by the Cochrane Bone, Joint and Muscle Trauma Group. A total of 19 trials were eligible: 18 used intravenous administration, one also evaluated oral dosing, and one trial evaluated topical use. TXA led to a significant reduction in the proportion of patients requiring blood transfusion [risk ratio [RR] 2.56, 95% confidence interval [CI] 2.1–3.1, P < 0.001; heterogeneity I2 = 75%; 14 trials, 824 patients]. Using TXA also reduced total blood loss by a mean of 591 mL [95% CI 536–647, P < 0.001; I2 = 78%; nine trials, 763 patients]. The clinical interpretation of these findings is limited by substantial heterogeneity. However, subgroup analysis of high-dose [> 4 g] TXA showed a plausible consistent reduction in blood transfusion requirements [RR:5.33; 95% CI 2.44–11.65, P < 0.001; I2 = 0%], a finding that should be confirmed by a further well-designed trial. The current evidence from trials does not support an increased risk of deep-vein thrombosis [13 trials, 801 patients] or pulmonary embolism [18 trials, 971 patients] due to TXA administration. When our study was combined with the previously mentioned trials, it demonstrated the safety of intra-articular acid injections because the medication is given locally, requires a modest amount, and is not absorbed systemically, lowering the likelihood of complications.
Compared to intravenous administration, intra-articular administration of TXA seems to be more effective in terms of reducing blood loss and transfusion frequency. Administration of TXA may improve the general condition of patients with TKA by maintaining a hemodynamically stable state, aiding in recovery, and reducing the chance of transfusion-associated side effects and complications.
Intra-articular TXA offers a significant advantage in reducing perioperative blood loss in TKA without added risks of thromboembolic events, potentially improving recovery and decreasing post-operative complications.
References
- 1.Liu D, Dan M, Martos SM, Beller E. Blood management strategies in total knee arthroplasty. Knee Surg Relat Res 2016;28:179. [Google Scholar]
- 2.Spahn DR, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, et al. Management of bleeding and coagulopathy following major trauma: An updated European guideline. Crit Care 2013;17:R76. [Google Scholar]
- 3.Bidolegui F, Arce G, Lugones A, Pereira S, Vindver G. Tranexamic acid reduces blood loss and transfusion in patients undergoing total knee arthroplasty without tourniquet: A prospective randomized controlled trial. Open Orthop J 2014;8:250. [Google Scholar]
- 4.Mannucci PM. Hemostatic drugs. N Engl J Med 1998;339:245-53. [Google Scholar]
- 5.McCormack PL. Tranexamic acid: A review of its use in the treatment of hyperfibrinolysis. Drugs 2012;72:585-617. [Google Scholar]
- 6.Roy SP, Tanki UF, Dutta A, Jain SK, Nagi ON. Efficacy of intra-articular tranexamic acid in blood loss reduction following primary unilateral total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 2012;20:2494-501. [Google Scholar]
- 7.Ishida K, Tsumura N, Kitagawa A, Hamamura S, Fukuda K, Dogaki Y, et al. Intra-articular injection of tranexamic acid reduces not only blood loss but also knee joint swelling after total knee arthroplasty. Int Orthop 2011;35:1639-45. [Google Scholar]
- 8.Seo JG, Moon YW, Park SH, Kim SM, Ko KR. The comparative efficacies of intra-articular and IV tranexamic acid for reducing blood loss during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 2013;21:1869-74. [Google Scholar]
- 9.Sa-Ngasoongsong P, Channoom T, Kawinwonggowit V, Woratanarat P, Chanplakorn P, Wibulpolprasert B, et al. Postoperative blood loss reduction in computer-assisted surgery total knee replacement by low dose intra-articular tranexamic acid injection together with 2-hour clamp drain: A prospective triple-blinded randomized controlled trial. Orthop Rev 2011;3:e12. [Google Scholar]
- 10.Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: A systematic review and meta-analysis. J Bone Joint Surg Br 2011;93:1577-85. [Google Scholar]