Considering the morbidity experienced by the patients of avascular necrosis of bones, vascularized bone transfer should primarily be considered for the younger patients with AVN, with an option of definitive arthroplasty, kept in reserve later in their lives, if required.
Dr. Mainak Mallik, Department of Burns and Plastic Surgery, All India Institute of Medical Sciences, Bhubaneswar, Odisha, India, Department of Burns and Plastic Surgery, All India Institute of Medical Sciences, Kalyani, West Bengal, India. E-mail: mognomainak@gmail.com
Introduction: Avascular necrosis of bone, due to various etiologies, is not an uncommon skeletal pathology seen in clinical practice. Treatment of these conditions includes treating the resultant pain, stiffness, and limitations of movement surgically with bone grafts both vascularized and non-vascularized and arthroplasty in most advanced stages.
Materials and Methods: In a duration of 2 years, five patients were treated for such pathologies using vascularized bones (pedicled or free). All of them were subjected to radioisotope (Technetium 99m) scan postoperatively to evaluate the viability of the transferred bones. The post-operative pain was assessed using visual analog scale (VAS) to assess the outcome of treatment by comparing the score before and after surgery.
Results: All patients improved symptomatically as per the outcome assessment with VAS score with viability of the transferred bones as evidenced by radioisotope scan.
Conclusion: Vascularized bone transfer is indispensable in the treatment of avascular necrosis of bone and the complications thereof, and should always be offered primarily to younger patients especially.
Keywords: Avascular necrosis of bone, vascularized bone graft, osteonecrosis of femoral head, scaphoid fracture non-union.
Avascular necrosis (AVN) of bone, affecting various components of human skeletal system, is not uncommon in clinical practice. Commonly involved regions include the epiphyses of long bones at the weight-bearing site. Head of the femur is most commonly involved, followed by knee, talus, and small bones of wrist, such as lunate and scaphoid. AVN results from a myriad of causes, of which, common ones include trauma, chemo-radiation, and hematological disorders such as coagulopathies and sickle cell disease. Corticosteroid intake is also one of the common causes of this pathology. Treatment modality of this condition, by and large, is governed by the stage of the disease. It varies from conservative management with non-steroidal anti-inflammatory drugs to advanced form of surgical management, like arthroplasties. In younger patients with advanced stage of AVN affecting major joints, arthroplasty is not considered ideal, as the procedure most often needs to be repeated after about two decades. Hence, for these groups of patients, debridement of necrotic bone and replacing it with vascularized bone graft is considered to be the most appropriate treatment to alleviate the symptoms of pain, stiffness, and limitations of movement and also prevent further complications. This is a short retrospective single-center series of cases of AVN managed by vascularized bone transfer.
This is a retrospective, descriptive study of the patients suffering from AVN of bone who presented to the department of plastic surgery after being referred from the orthopedics department of a single tertiary care teaching institute during 2 years, from April 2021 to March 2023. The patients requiring bone grafts for reasons other than AVN of bone, those not treated by vascularized bone graft, and those who did not follow-up for a minimum period of 6 months were excluded from this study. The severity of the disease (type and stage), in cases of osteonecrosis of femoral head (ONFH) due to AVN, was determined by the radiological evaluations, which included plain radiograph and the CT scans with 3D reconstruction, as per the classification recommended by Japanese Investigation Committee (JIC) as described in Table 1. [1]
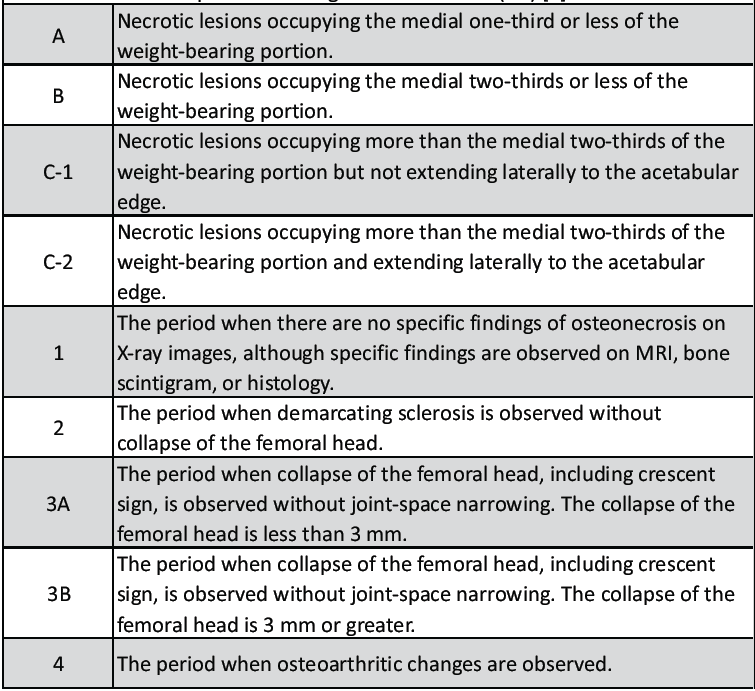
Table 1: Classification of ONFH (osteo-necrosis of femoral head) as per Japanese Investigation Committee (JIC) [1]
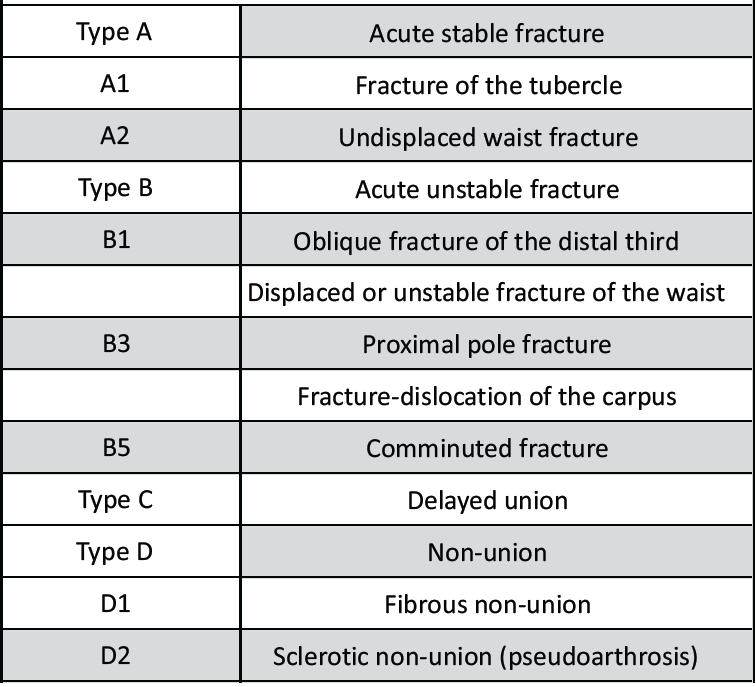
Table 2: Classification of scaphoid fracture as per Herbert and Fisher classification [2]
Every work related to this study was done in compliance with the Declaration of Helsinki (1975) and its subsequent modifications, till date. As it is a retrospective, descriptive study involving techniques, already as per published literature, statement of IRB has not been included. There was no identification of human subjects and no disclosure of protected healthcare information, such as a patient’s name, initials, hospital number, or date of birth. Informed consent was taken in every case, including consent for using clinical and operative images (without including any identifiable traits like tattoos), after explaining the procedure details pertaining to the specific pathology and explaining any alternative treatment, if feasible for the same.
During the study period of 2 years, a total of five patients who received treatment for AVN of bone were included as per the inclusion criteria. Out of them, three presented with necrosis of femoral head and two with non-union of fracture scaphoid (Table 3).
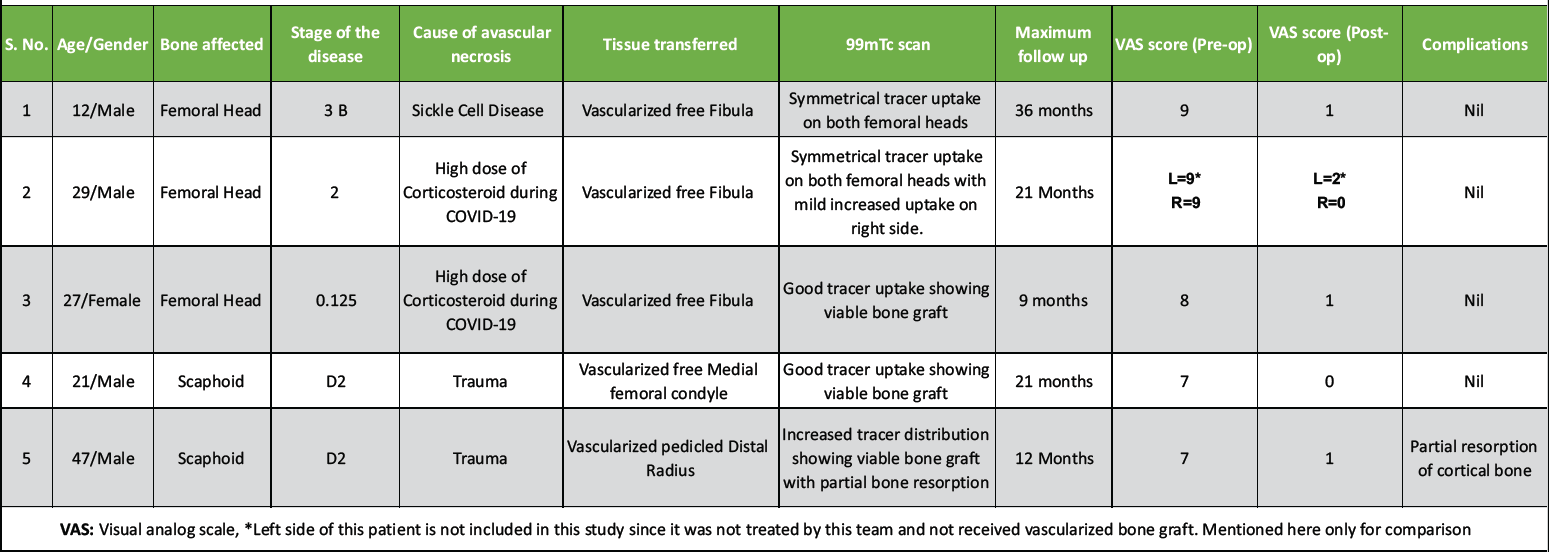
Table 3: The details of patients treated
Four of the patients were males and one female. The minimum age of the patients was 12 years and maximum 47 years with a mean age of 24.8 years. Out of the three patients having ONFH, the first one had type B stage 2, second one type C1 stage 3A, and the third one type C2 stage 3B disease. Out of the three ONFH cases, one patient was a sufferer of sickle cell disease and the other two patients were the sequelae of high intake of corticosteroid drugs received during treatment of COVID 19. All three patients with ONFH were treated with core decompression of necrotic bone and replacement with vascularized segments of fibula (Figs. 1, 2, 3).
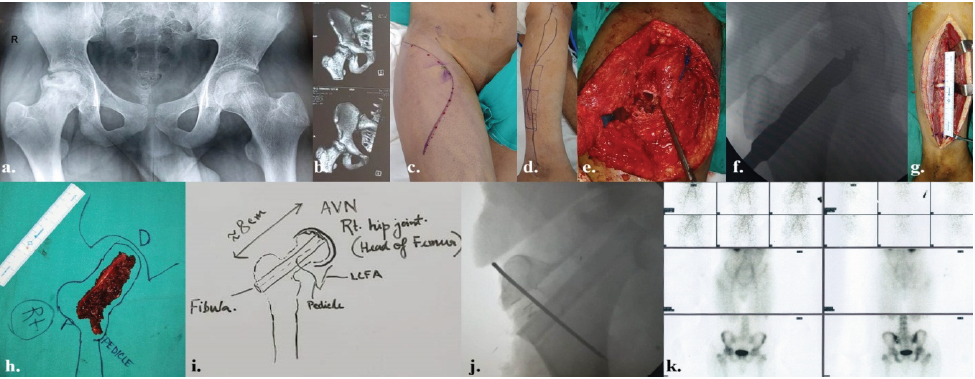
Figure 1: The details of rt. osteonecrosis of femoral head in a 12-year-old male as a sequela to sickle cell disease – (a) pre-operative skiagram pelvis anteroposterior view; (b) pre-operative computed tomography-scan with 3D reconstruction of rt. Pelvis; (c) pre-operative marking of incision on rt. Hip and upper thigh; (d) pre-operative marking of free fibula vascularized bone on rt. leg; (e) intra-operative coring of rt. Femoral head; (f) intra-operative image intensifier view of the reamer inside the rt. Femoral head; (g) intraoperative fibula harvest; (h) planning and orientation of fibula after flap detachment; (i) diagrammatic planning intra-operatively; (j) intraoperative picture of image intensifier after introduction of fibula and fixation with K-wire; and (k) Tc99m scan after 3 months showing symmetrical uptake of tracers on both femoral heads.
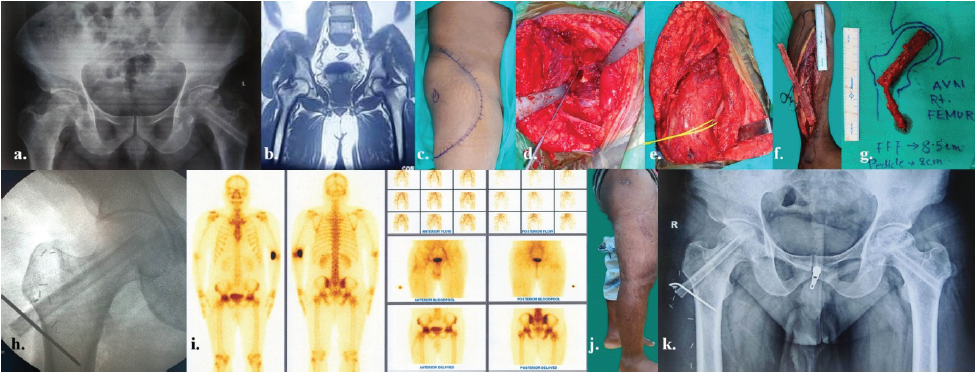
Figure 2: The details of rt. osteonecrosis of femoral head in a 29-year-old male as a sequela to high dose of corticosteroid during COVID-19 – (a) pre-operative skiagram pelvis anteroposterior view with previously operated lt. AVN femur with non-vascularized bone graft done outside, presently complaining of pain in rt. hip; (b) pre-operative computed tomography-scan of pelvis; (c) pre-operative marking of incision on rt. Hip and upper thigh; (d) intra-operative coring of rt. Femoral head; (e) intra-operative picture of recipient vessel- descending branch of circumflex femoral vessels, dissected and looped in yellow tape; (f) intraoperative fibula harvest; (g) planning and orientation of fibula after flap detachment; (h) intraoperative picture of image intensifier after introduction of fibula and fixation with K-wire; (i) Tc99m scan after 3 months showing symmetrical uptake of tracers on both femoral heads with mild increased uptake on right side; (j) Post-operative follow-up after 3 months; and (k) post-operative follow-up skiagram pelvis AP view after 3 months showing bone uptake.
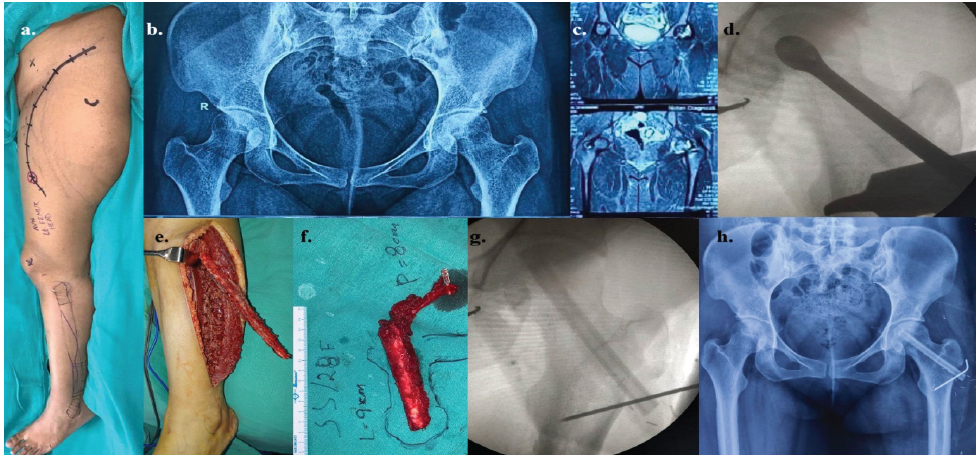
Figure 3: The details of lt. osteonecrosis of femoral head in a 27-year-old female as a sequela to high dose of corticosteroid during COVID-19 – (a) pre-operative picture of the lt. hip and lower extremity with the marked incisions; (b) pre-operative skiagram pelvis anteroposterior (AP) view; (c) pre-operative computed tomography-scan of pelvis; (d) intraoperative image intensifier view of the reamer inside the lt. Femoral head; (e) intraoperative fibula harvest; (f) planning and orientation of fibula after flap detachment; (g) intraoperative picture of image intensifier after introduction of fibula and fixation with K-wire; and (h) post-operative follow-up skiagram pelvis AP view after 3 months.
Both patients of non-union of scaphoid fracture were of type D1 as per Herbert and Fisher classification, as sequelae to trauma, with fractures involving waist of scaphoid. In one of them, the necrotic bone was substituted with vascularized medial femoral condyle (MFC) by microvascular transfer and the other, a segment of pedicled distal radius based on 1,2 intercompartmental supra-retinacular artery (ICSRA) (Fig. 4).
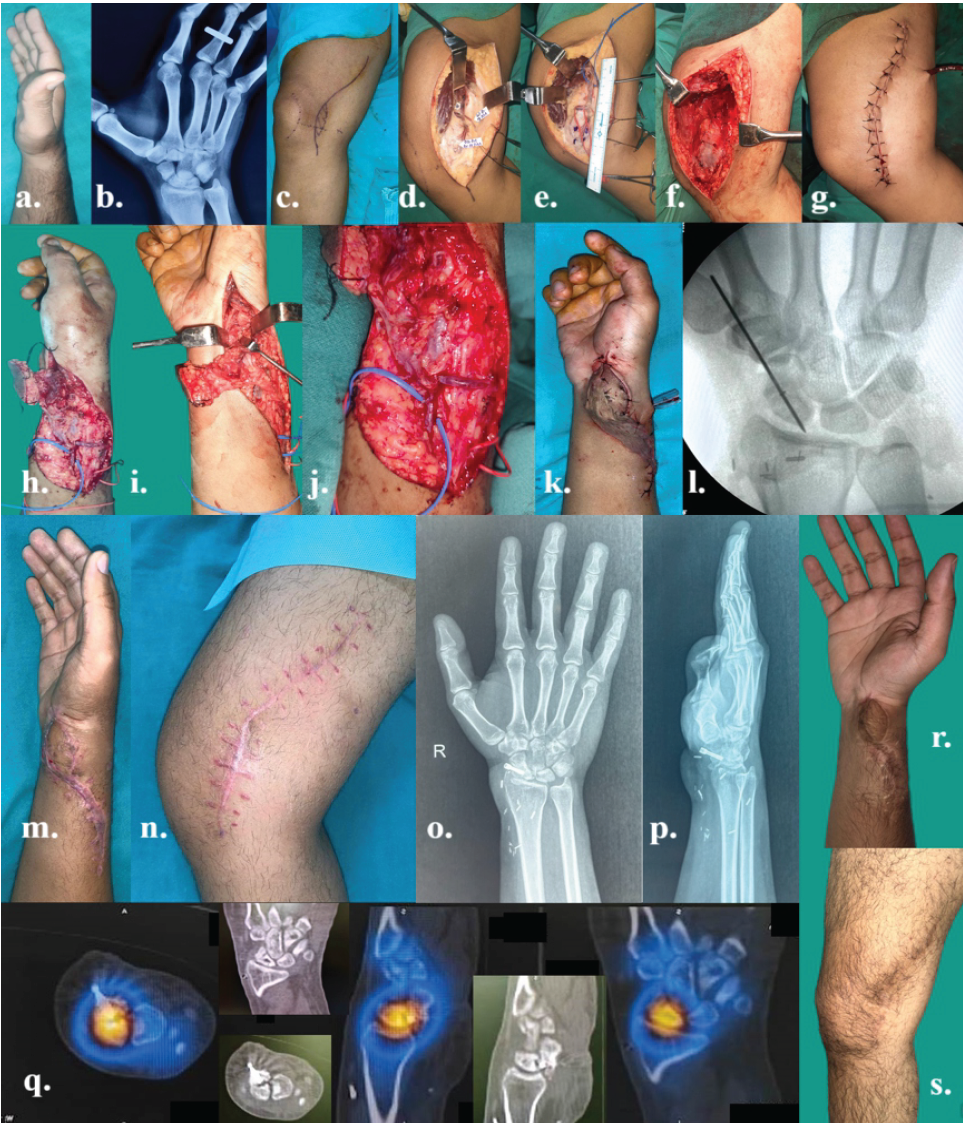
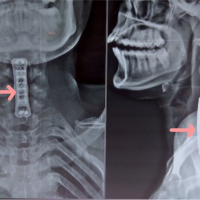
Figure 4: The details of AVN scaphoid of rt. hand in a 21-year-old male – (a) pre-operative picture of rt. hand; (b) pre-operative skiagram anteroposterior (AP) view of rt. hand with wrist joint; (c) pre-operative marking of vascularized rt. Medial femoral condyle (MFC) flap; (d-g) intraoperative vascularized MFC flap harvest and closure of donor area; (h-j) osteocutaneous flap inset after exploration of rt. scaphoid and recipient vessels looped in blue tape; (k) after flap inset and remnant of defect closed with skin graft; (l) image intensifier view of fixation with Herbert screw (permanent) and temporary single K-wire; (m and n) post-operative views of recipient and donor areas after 6 weeks; (o and p) 6 weeks post-operative views of skiagram (AP and lateral) rt. hand; (q) Tc99m scan after 6 weeks showing good uptake of tracers indicating viable bone transfer; and (r and s) long term post-operative pictures of rt. hand and rt. knee donor area after 6 months.
In the first case, the fracture site was exposed through volar approach and the bone fixation was performed using a single Herbert’s screw. Whereas in the second case, the approach was dorsal and the bone graft was fixed with a percutaneous Kirschner wires (Fig. 5). The follow-up period ranged from 9 to 36 months with a mean of 19.8 months. No surgical complication was observed in any of the patients. Partial resorption of cortical bone was observed in one case of AVN and non-union of fracture scaphoid where pedicled vascularized distal radius was used (Fig. 5).
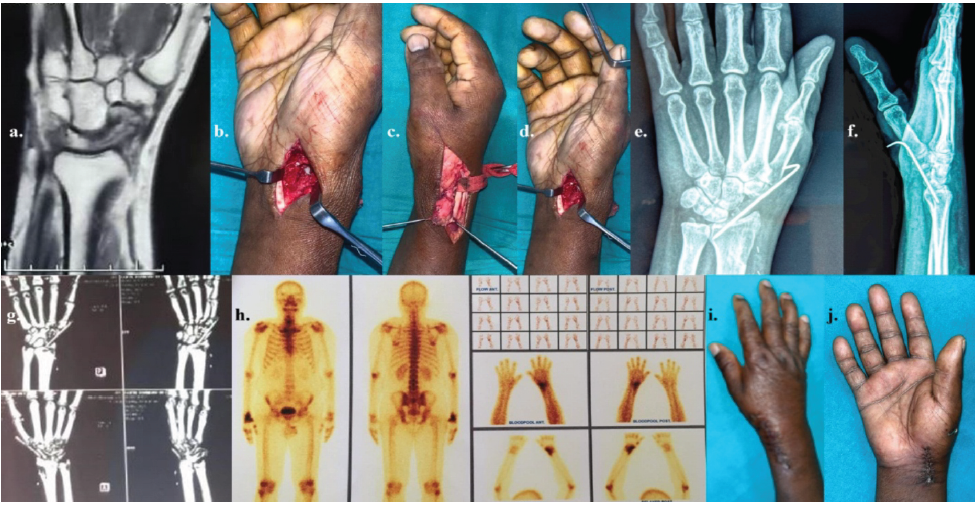
Figure 5: The details of AVN scaphoid of rt. hand in a 47-year-old male – (a) pre-operative computed tomography (CT) scan of rt. Wrist; (b) surgical approach to rt. Scaphoid and defect after debridement of necrotic bone; (c) harvest of vascularized segment of rt. distal radius based on 1,2 intercompartmental supra-retinacular artery; (d) transfer of pedicled distal radius to the defect in scaphoid; (e and f) post-operative radiograph showing bone graft fixed with K-wire; (g) CT-scan images with 3D reconstruction rt. Wrist in 6 weeks post-operative follow-up period showing partial resorption of bone; (h) Tc99m scan after 6 weeks showing increased uptake of tracers indicating viable bone transfer; and (i and j) showing post-operative 2-month follow-up.
The minimum pre-operative pain score was 7 and the maximum 9 with a mean pain score of 8. The post-operative pain score varied from 0 to 1 with a mean of 0.6. Post-operative 99mTc bone scan demonstrated good tracer uptake in all cases indicating good vascularity of bone grafts.
AVN of bone is commonly encountered after trauma to femoral head, humeral head, and small bones such as talus and scaphoid, but less often being referred to a reconstructive surgeon for replacement with vascularized bone graft. The dead bone resorbs or undergoes fibrosis, which may show radiolucency or sclerosis in radiographs. Specific type of fractures in these bones makes them vulnerable to vascular deprivation and subsequent osteonecrosis, which may proceed to worsen the situation such as non-union and collapse of the joints. The incidence of the ONFH varies from 14.7% to 45% with an average incidence of 17.3% [3]. In this study, the staging of the ONFH cases was done as per the recommendations of revised JIC, although there are other classifications used to categorize this disease and plan for treatment [4]. This classification was used as it is a retrospective study using plain radiograph and computed tomography scan to diagnose and stage the disease for treatment planning. Some of the classification systems have become outdated and do not include modern imaging modalities, whereas others need more invasive modalities like contrast MRI [1,4,5,6]. Similarly, the incidence of non-union fracture scaphoid due to AVN may range from 13% to 50% mostly when the fracture involves the scaphoid waist [3,7,8]. Proximal pole of scaphoid is usually affected by AVN whereas rare cases have also been reported to involve the distal pole [9].
AVN of bone may also result from cytotoxic agents, disruption of vascular system, extravascular compression, and intravascular occlusion of blood flow. Clinical causes include specific fractures, sickle cell disease, chemotherapeutic agents, and high dose of corticosteroids among others. Genetic factors may play a role, but a large proportion of the cases are idiopathic [10]. In our series, one ONFH was due to sickle cell disease and the other two were due to high dose of corticosteroids received for treatment of COVID-19 during the pandemic. Both the cases of AVN of scaphoid were due to traumatic fracture involving the waist of scaphoid, of type D2 as per Herbert and Fisher classification. Complete fracture at this region results in disruption of dorsal carpal artery, a branch of radial artery which is responsible for the vascular supply to the proximal segment of scaphoid, leading to AVN and non-union of fracture.
Surgical treatment is the mainstay of treatment of ONFH, although conservative management in the form of adipose-tissue derived stem cells or the platelet-rich plasma has shown some promise for the very early stage of the disease (Stage – 1) [11]. Early stages of ONFH are treated by debridement of necrotic or sclerotic bone performed by core decompression and refilling the part with vascularized or non-vascularized bone grafts. This treatment modality has proved to reduce the symptoms, preventing further progression of the disease process, whereas for advanced ONFH above 40 years of age, hip arthroplasty is the treatment of choice in most cases [11]. Core decompression of femoral head is usually followed by filling the cavity with cortico-cancellous bone graft. Although both non-vascularized as well as vascularized bone grafts are used to fill the cavity left after core decompression, vascularized bone graft had been observed to yield better long-term clinical outcome due to lesser resorption [10,12]. In all three of our cases of ONFH, we had a similar approach (Figs. 1, 2, and 3). Core decompression was performed with rimmer to prepare the diameter of the cavity in the femoral head just adequate to accommodate the fibula. After harvesting the fibula of appropriate length, periosteum was sutured at both the ends to prevent it being stripped of while tapping into the femoral neck. After insertion into the cavity made in the femoral neck and head, the fibula was stabilized with a percutaneous K-wire introduced through its lateral end under the control of image intensifier, with utmost care to avoid injury to the peroneal vessel pedicles. The peroneal artery and venae comitantes were anastomosed end to end with the descending branch of lateral circumflex femoral vessels (pre-dissected and looped) to establish the vascularity of bone flap. Postoperatively, anastomotic flow was monitored with the help of an 8 MHz hand held Doppler, through the skin marked overlying the anastomosis. Although implantable Doppler probe would have been the best method of flap monitoring in such cases, in resource crunch circumstances, hand held Doppler was considered as an alternative (Fig. 4). In all of our cases, we could achieve good symptomatic relief, till the point of last follow-up (Mean follow-up period of 19.8 months). One of these patients had bilateral severe disease and underwent non-vascularized fibula graft for one side previously in a different set up. We treated his contralateral side with a vascularized fibula graft (Fig. 2). His pain score came down from 9 (Pre-op) to 0 (post-op) on the right side which received vascularized bone whereas the change was from 9 (pre-operative) to 2 (post-operative) on the side which received non-vascularized bone. This observation corroborated to that of Kim et al. [13] We documented the vascularity of the bone grafts by radioisotope (99mTc) bone scan in all the cases after the surgical sites healed completely and the patients became ambulatory.
We tried to replace the necrotic part of scaphoid with vascularized bone graft which is the treatment of choice for these cases [14,15,16]. Pedicled distal radius, based on 1,2 ICSRA and free vascularized MFC, have been the preferred donor sites for this purpose [17,18]. Out of the two cases of non-union of scaphoid fracture treated by us, the distal radius was used in one and free vascularized MFC based on descending genicular artery and its venae comitantes was used in the other patient. The recipient vessels for free vascularized MFC were radial artery (end-to-side with descending genicular artery) and the cephalic vein (end-to-end with the venae comitantes). MFC has been observed to offer better outcome for this purpose in some of the reported series [19,20,21,22]. Although only two cases of non-union scaphoid with AVN were managed by us, a partial resorption of bone was observed in the post-operative radiographs where distal radius was used. We could also find a perforator arising from the descending genicular artery supplying overlying skin of the MFC, which we used as a marker for monitoring the flap during early post-operative period by transferring this skin island along with the bone. We had treatment outcomes comparable to most of the reports published in literature.
AVN of bone leading to ONFH and non-union of fracture scaphoid is not uncommon, but difficult to treat. High index of suspicion should be there in the treating surgeons’ mind while evaluating these cases. For better management of these patients, the concerned orthopedic team should always collaborate with a microvascular reconstructive surgeon in an inter-disciplinary team approach, to provide a vascularized bone graft from ideal sources.
Limitations of this study
This is a short clinical case series of very small sample size. Statistical analysis was precluded due to inadequacy of ordinal data collected from this study. Furthermore, no other modality of treatment or other source of bone graft was used in the cases of ONFH to compare the outcome of the treatment. Hence, the observations of this study need to be supported by studies recruiting larger sample sizes and comparison between different sources of bone grafts in terms of more objective parameters.
This study corroborates the importance of vascularized bone graft in the treatment modality for cases with AVN involving any part of the skeletal system. It offers long term relief of the symptoms these patients experience. The procedure helps in better union of non-united fracture of small bones and in larger bones like femoral head, it delays the arthroplasty for a significant period of time, particularly in younger patients. However, the conclusion drawn from this series need to be validated further by better designed studies incorporating larger sample size.
Vascularized bone transfer offers safe, reliable, and durable relief from symptoms, to the patients suffering from AVN of bone hence, should be considered as the treatment of choice primarily in younger patients, with the option of definitive arthroplasty, if required, later in their lives.
References
- 1. Sugano N, Atsumi T, Ohzono K, Kubo T, Hotokebuchi T, Takaoka K. The 2001 revised criteria for diagnosis, classification, and staging of idiopathic osteonecrosis of the femoral head. J Orthop Sci. 2002;7(5):601-5. doi: 10.1007/s007760200108. PMID: 12355139. [Google Scholar] [PubMed] [CrossRef]
- 2. Herbert TJ, Fisher WE. Management of the fractured scaphoid using a new bone screw. J Bone Joint Surg Br. 1984 Jan;66(1):114-23. doi: 10.1302/0301-620X.66B1.6693468. PMID: 6693468. [Google Scholar] [PubMed] [CrossRef]
- 3. Large TM, Adams MR, Loeffler BJ, Gardner MJ. Posttraumatic Avascular Necrosis After Proximal Femur, Proximal Humerus, Talar Neck, and Scaphoid Fractures. J Am Acad Orthop Surg. 2019 Nov 1;27(21):794-805. doi: 10.5435/JAAOS-D-18-00225. PMID: 31149969. [Google Scholar] [PubMed] [CrossRef]
- 4. Kuroda Y, Tanaka T, Miyagawa T, Kawai T, Goto K, Tanaka S, Matsuda S, Akiyama H. Classification of osteonecrosis of the femoral head: Who should have surgery? Bone Joint Res. 2019 Nov 2;8(10):451-458. doi: 10.1302/2046-3758.810.BJR-2019-0022.R1. PMID: 31728183; PMCID: PMC6825048. [Google Scholar] [PubMed] [CrossRef]
- 5. Ficat RP (1985) Idiopathic bone necrosis of the femoral head. Early diagnosis and treatment. J Bone Joint Surg Br 67:3–9. [Google Scholar] [PubMed]
- 6. Gardeniers JW (1992) A new international classification of osteonecrosis of the ARCO Committee on terminology and classification. J Jpn Orthop Assoc 66:18–20. [Google Scholar] [PubMed]
- 7. Cooney WP, Dobyns JH, Linscheid RL. Fractures of the scaphoid: A rational approach to management. Clin Orthop Relat Res 1980;149:90 7. [Google Scholar] [PubMed]
- 8. Freedman DM, Botte MJ, Gelberman RH. Vascularity of the carpus. Clin Orthop Relat Res 2001;383:47 59. [Google Scholar] [PubMed]
- 9. Kapoor S, Pawar I, Kapoor S. Posttraumatic osteonecrosis and nonunion of distal pole of scaphoid. Indian J Orthop. 2013 Jul;47(4):425-8. doi: 10.4103/0019-5413.114941. PMID: 23960291; PMCID: PMC3745701. [Google Scholar] [PubMed] [CrossRef]
- 10. Matthews AH, Davis DD, Fish MJ, Stitson D. Avascular Necrosis. 2023 Aug 28. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. PMID: 30725692. [Google Scholar] [PubMed]
- 11. Pak J, Lee JH, Jeon JH, Lee SH. Complete resolution of avascular necrosis of the human femoral head treated with adipose tissue-derived stem cells and platelet-rich plasma. J Int Med Res. 2014 Dec;42(6):1353-62. doi: 10.1177/0300060514546940. Epub 2014 Oct 3. PMID: 25281062. [Google Scholar] [PubMed] [CrossRef]
- 12. Millikan PD, Karas V, Wellman SS. Treatment of osteonecrosis of the femoral head with vascularized bone grafting. Curr Rev Musculoskelet Med. 2015 Sep;8(3):252-9. doi: 10.1007/s12178-015-9285-8. PMID: 26068178; PMCID: PMC4596199. [Google Scholar] [PubMed] [CrossRef]
- 13. Kim SY, Kim YG, Kim PT, Ihn JC, Cho BC, Koo KH. Vascularized compared with nonvascularized fibular grafts for large osteonecrotic lesions of the femoral head. J Bone Joint Surg Am. 2005 Sep;87(9):2012-8. doi: 10.2106/JBJS.D.02593. PMID: 16140817. [Google Scholar] [PubMed] [CrossRef]
- 14. Tambe AD, Cutler L, Stilwell J, Murali SR, Trail IA, Stanley JK. Scaphoid non-union: the role of vascularized grafting in recalcitrant non-unions of the scaphoid. J Hand Surg. 2006;31B:185–190. [Google Scholar] [PubMed]
- 15. Kirkeby L, Baek Hansen T. Vascularised bone graft for the treatment of non-union of the scaphoid. Scand J Plast Reconstr Surg Hand Surg. 2006;40:240–243. [Google Scholar] [PubMed]
- 16. Kawamura K, Chung KC. Treatment of scaphoid fractures and nonunions. J Hand Surg Am. 2008 Jul-Aug;33(6):988-97. doi: 10.1016/j.jhsa.2008.04.026. PMID: 18656779; PMCID: PMC4405116. [Google Scholar] [PubMed] [CrossRef]
- 17. Zaidemberg C, Siebert JW, Angrigiani C. A new vascularized bone graft for scaphoid nonunion. J Hand Surg. 1991;16A:474–478. [Google Scholar] [PubMed]
- 18. Yamamoto H, Jones DB, Moran SL, Bishop AT, Shin AY. The arterial anatomy of the medial femoral condyle and its clinical implications. J Hand Surg Eur 2010;35(7):569–574. [Google Scholar] [PubMed]
- 19. Sakai K, Doi K, Kawai S (1991) Free vascularized thin corticoperiosteal graft. Plast Reconstr Surg 87, 290–298. [Google Scholar] [PubMed]
- 20. Koriem I, Agina AA & El Ghazawy AK (2023) Treatment of failed scaphoid nonunion fixation using free medial femoral condyle vascularized bone grafting. SICOT-J 9, 7 [Google Scholar] [PubMed]
- 21. Pulos N, Kollitz KM, Bishop AT, Shin AY. Free Vascularized Medial Femoral Condyle Bone Graft After Failed Scaphoid Nonunion Surgery. J Bone Joint Surg Am. 2018 Aug 15;100(16):1379-1386. doi: 10.2106/JBJS.17.00955. PMID: 30106819. [Google Scholar] [PubMed] [CrossRef]
- 22. Kumta S, Warrier S, Jain L, Ummal R, Menezes M, Purohit S. Medial femoral condyle vascularised corticoperiosteal graft: A suitable choice for scaphoid non-union. Indian J Plast Surg. 2017 May-Aug;50(2):138-147. doi: 10.4103/ijps.IJPS_62_17. PMID: 29343888; PMCID: PMC5770926. [Google Scholar] [PubMed] [CrossRef]