Quantitative Ultrasound of Calcaneum may be utilized in symptomatic patients as alternative to DEXA for BMD assessment.
Dr. Nitish Kumar, Department of Orthopedics, All India Institute of Medical Sciences, Gorakhpur, Uttar Pradesh, India. Email: nitishaiims@gmail.com
Introduction: Although osteoporosis can be diagnosed by many methods like quantitative ultrasound (QUS), peripheral dual-energy X-ray absorptiometry (DEXA), hand X-rays, or clinical examinations but the diseases remain undertreated mainly due to a lack of mechanism to identify individuals who may be at high risk of fracture and might get benefit from the timey interventions. The availability of central DEXA is not uniform in many countries; hence, a reliable, cheap, portable handy equipment is needed. Role of QUS as an alternative to DEXA has been studied for selective population but a very few studies have attempted its utility in the general population for routine use.
Materials and Methods: The cross-sectional observational study was done with 283 patients with age more than 40 years with both sexes. Any patient with a history of trauma, infection, surgical infection or immobilization of the lower limb for more than 6 weeks in the preceding 1 year. The left calcaneum was used for calculating T-score by QUS by utilizing 500 kHz ultrasonic frequency for the ultrasound pulse penetration method.
Results: The mean age of the study population was 51.4 ± 10.54 years with 188 female and 95 males. The prevalence of osteopenia was slightly higher among the female population (68.6%) in the current study group in comparison to males (54.7%). The age and symptoms of osteopenia were the two variables which significantly determined the predictive power of QUS in diagnosing osteopenia.
Conclusion: The use of calcaneum QUS proved to be a rapid and efficient method for assessing bone mass density, demonstrating its ability to detect osteopenia, especially in older and symptomatic patients. Given its cost-effectiveness, portability, and safety due to the absence of ionizing radiation, QUS serves as a valuable and attractive alternative for initial bone health assessment, particularly in settings with limited access to central DEXA.
Keyword: Quantitative ultrasound, bone mineral density, dual-energy X-ray absorptiometry, calcaneum.
Osteoporosis is caused by disorientation of bone tissue micro-architecture, causing decrease in bone strength, resulting in increased risk of fracture. These fractures increase morbidity and mortality of the elderly population and are commonly due to hip and spine fractures. The early diagnosis and use of Food and Drug Administration-approved drugs can increase the bone mass density and hence can reduce the risk of fracture [1]. The current diagnostic protocol employs either clinical diagnosis by documenting fracture of spine/hip following trivial trauma or the use of dual-energy X-ray absorptiometry (DEXA) to detect low bone mass density [2].
Although there are many standard methods for diagnosing osteoporosis but the disease remains undertreated [3]. The International Osteoporosis Foundation in its report in 2008 have found that more than 2/3rd people at risk for osteoporosis were never diagnosed or screened for it, hence never got treatment [4]. One of the important causes for under treatment is a lack of mechanism to identify individuals who may be at high risk of facture and might get benefit from the timely interventions. Value of bone mineral density (BMD) indicates bone strength and fracture but the risk for fracture is confounded by advanced age and history of previous fractures [5].
The availability of central DEXA is not uniformly available in many countries, and further high cost associated with it makes its routine use difficult [6]. Due to these problems, many researchers are adopting low-cost and easily available diagnostic modalities such as quantitative ultrasound (QUS), peripheral DEXA, hand X-rays, or clinical examinations, like use of FRAX fracture prediction tool (Centre for Metabolic Bone Diseases, University of Sheffield, UK) to replace central DEXA [7].
The QUS can be employed to various bones; however, due to a lack of technical guidelines and its interpretation for clinical use for individual use makes it less popular despite having many advantages over DEXA [8]. The QUS has a low-cost per test, equipment is small, handy, portable and cheap [9]. There is no need for a dedicated technician to run the test, and complete lack of radiation hazards gives it an extra edge over DEXA.
Heel QUS has shown promising feature in the diagnosis of osteoporosis, hence fragility fractures [10]. The predictive power of this test is greater at lower values for osteoporotic fractures.
There are studies to assess the role of QUS in selective populations, like suffering from particular diseases or in female, but a very few studies have aimed toward its utility in the general population [11,12]. Hence, this study was design to find utility of QUS for BMD assessment in general population.
This cross-sectional observational study was done at a university level teaching hospital. A total of 283 patients who had visited the out-patient department of orthopedics department with various complaints were included for this study.
The criteria for including patients in this study were –
- Age – 40 years or above
- Sex – Patients from both sexes
- Consenting to participate.
The patients with following parameters were excluded from this study
- Any history of foot injury,
- Foot infection
- Any surgical intervention done at foot
- History of plaster application or immobilization of limb for more than 6 weeks in preceding 1 year.
Once patients were recruited for this study, their height, weight, dietary habit and symptoms of osteopenia were recorded. Any patient with a history of non-specific back pain, back pain worsening on walking, a change of back posture or a history of fracture following low-velocity trauma was considered symptomatic patients.
The left calcaneum was used for calculating T-score by QUS. For this left heel was used to perform the QUS assessment by ultrasound bone densitometer which is compact, portable, all-in-one system with an inbuilt thermal printer. The machine uses calcaneum by the ultrasound pulse penetration method by utilizing speed of sound for determining bone mass density. The machine employs radiation-free ultrasound technology at a center frequency of 500 kHz, which is found to be safe for pregnant women and children. Total procedure took <30 s.
The P-value and multivariate regression analysis were done to assess factors having statistical significance in influencing T-score in the study population.
The mean age of the study population was 51.4 ± 10.54 years. The no. of participants in different age groups was 40–50 years (138 participants), 51–60 years (78 participants) more than 60 years (67 participants). The study population had 188 females and 95 males. The mean height for the participants was 157.05 ± 10.11 cm, weight 67.70 ± 10.8 kg, body mass index (BMI) 27.29 ± 4.38 kg/m2. Out of the total, 196 had mixed diet pattern and 87 had vegetarian diet. Based on the history of osteopenia, 145 were symptomatic and 138 patients were asymptomatic.
The P-value using Kruskal–Wallis test for different age groups was 0.00029, which suggested that significant statistical difference in T-score. The older persons had more osteopenia as compare to the younger population. The prevalence of osteopenia was slightly higher among the female population (68.6%) in the current study group in comparison to males (54.7%). However, this difference was statistically not significant (P = 0.18) (Table 1).
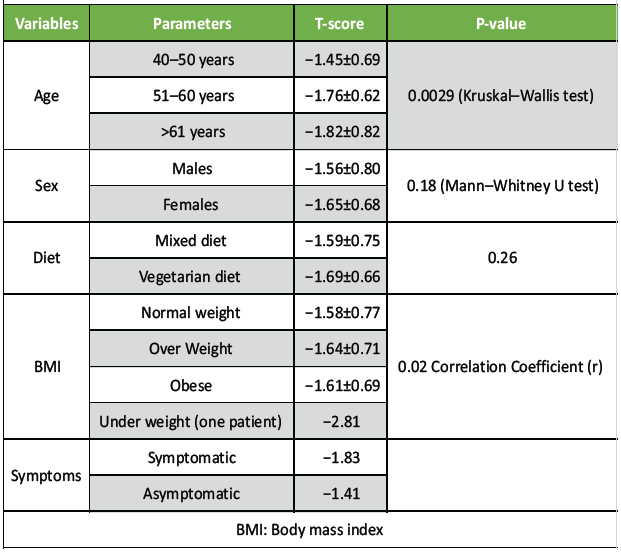
Table 1: P-value of study parameters
The dietary habit was not found to be an independent factor to influence bone mass. The P-value for both groups was 0.26. The patients with normal BMI had almost normal T-score, whereas the overweight population had slight osteopenia. The only underweight in this study population was significantly osteoporotic. The QUS was able to pick up osteopenia in symptomatic populations.
The multivariate regression analysis was done to find which all the factors were significantly influencing the T-score. Their P-value is tabulated here under (Table 2).
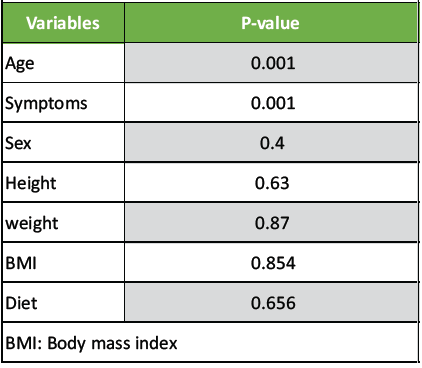
Table 2: The multivariate regression analysis
The age and symptoms of osteopenia were the two variables which significantly determined the predictive power of QUS in diagnosing osteopenia. The T-score was found to be less in older population. The relationship of age and T-score is depicted as under (Fig. 1). Similarly, the QUS study had showed that symptomatic patients had less T-score and it was statistically significant. All other variables did not influence the predictive power of this tool.
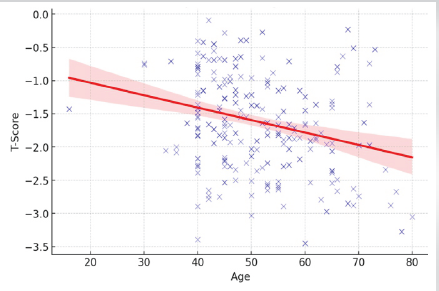
Figure 1: Relationship of age and T-score.
Receiver operating characteristic (ROC) curve suggests 73% sensitivity of the QUS in for BMD measurement (Fig. 2).
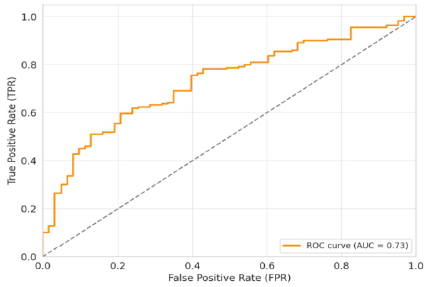
Figure 2: Receiver operating characteristic curve.
The health challenge posed by osteoporosis is due to a decrease in bone strength and an increased risk of fracture, causing morbidity and mortality among the elderly populations. QUS stands out as a promising technology over DEXA due to its wider and easy availability, which requires little inventory [9]. Further, it has demonstrated significant predictive value for fragility fractures secondary to osteoporosis, as well as hip fractures, comparable to other axial or peripheral bone strength measures [10].
The present study was aimed to understand the utility of QUS in a clinical setting by enrolling 283 patients aged 40 years or above from an orthopedic outpatient department. The methodology involved measuring T-scores using ultrasound pulse penetration on the left calcaneum [13]. The test was completed within <30 s.
The study population had a mean age of 51.4 ± 10.54 years, comprising 188 females and 95 males, which was comparable to the study by Kaushal et al. (50.0 ± 12.4 years) [14]. The findings of this study provide important insights into factors influencing bone mass density as assessed by QUS. A statistically significant difference in T-scores was observed across different age groups (P = 0.00029), with older individuals exhibiting more osteopenia compared to younger populations. This age-related decline in T-score was confirmed by multivariate regression analysis, which identified age as a significant determinant of the T-score (P = 0.001). While osteopenia was slightly more prevalent among females (68.6%) than males (54.7%) in the study group, this difference was not statistically significant (P = 0.18). The findings were similar to the study by Sharma et al. done on Indian population using calcaneal QUS and have found 75% of female and 50% of male to have lower BMD [15]. Dietary habits, whether mixed or vegetarian, did not show a statistically significant effect on osteopenia (P = 0.26). Regarding BMI, patients with normal weight generally had almost normal T-scores, whereas overweight individuals were slightly osteopenic; notably, the sole underweight patient in the study was significantly osteoporotic. However, the BMI was not found to be an independent predictor for osteoporosis. The same finding was elaborated by the study of Khinda et al. [16], suggesting higher BMI patients had less chance of getting osteoporosis. A key finding was that symptomatic patients had significantly lower T-scores than asymptomatic ones, and QUS was effectively able to detect osteopenia in these symptomatic individuals (P = 0.001 for symptoms in multivariate analysis). This suggests QUS’s potential as a valuable screening tool for patients presenting with symptoms indicative of osteopenia. Other variables such as sex, height, weight, and BMI were not found to significantly influence the predictive power of the QUS tool in this analysis. The ROC curve indicated a 73% sensitivity of QUS for BMD measurement, which is comparable to the study by Liu et al. [17]. Although integration of QUS results with clinical risk factors (CRFs) is not currently routine practice, prior studies, such as Hans et al., using data from the EPISEN cohort (n = 12,958 elderly women), demonstrated that combining QUS with CRFs (including BMI, fracture history, chair-rise test, falls, smoking, diabetes, and heel stiffness index) improves prediction of fracture risk over 5- and 10-year periods [18]. On multivariate analysis, they have found that apart from age, seven other variables had predictive value. Those included were BMI, any history of fracture, results of the chair test, a fall in the last 1 year, habit of cigarette smoking, diabetes mellitus, and the heel stiffness index. However, in the present study, the BMI was not found to be a significant predictor of osteopenia. The combination of CRFs and heel QUS was useful in predicting risk of fracture for different age groups, and it had better predictive value than the singular use of either CRFs or QUS.
This study reinforces the utility of QUS as a practical, low-cost, and accessible tool for preliminary bone health assessment. Its demonstrated ability to detect osteopenia, particularly in older and symptomatic populations, suggests it can play a crucial role in addressing the underdiagnosis of osteoporosis, especially in regions where central DXA access is limited.
This study concludes that calcaneum QUS proved to be a rapid and efficient method for assessing bone mass density when used for the general population. Given its cost-effectiveness, portability, and safety due to the absence of ionizing radiation, QUS serves as a valuable and attractive alternative for initial bone health assessment, particularly in settings with limited access to central DEXA and can help in the treatment of osteoporosis by early diagnosis of individuals at risk of fragility fractures.
Limitation of the study
The study suggested QUS as a valuable tool in diagnosing osteoporosis; however, a multicentric study with a larger population will be needed for validation.
QUS of calcaneum may be utilized in symptomatic patients as an alternative to DEXA for BMD assessment.
References
- 1. De Souza MP. Osteoporosis diagnosis and treatment. Rev Bras Ortop 2015;45:220-9. [Google Scholar] [PubMed]
- 2. Blake GM, Fogelman I. The role of DXA bone density scans in the diagnosis and treatment of osteoporosis. Postgrad Med J 2007;83:509-17. [Google Scholar] [PubMed]
- 3. Miller PD. Underdiagnosis and undertreatment of osteoporosis: The battle to be won. J Clin Endocrinol Metab 2016;101:852-9. [Google Scholar] [PubMed]
- 4. Sözen T, Özışık L, Başaran NÇ. An overview and management of osteoporosis. Eur J Rheumatol 2017;4:46-56. [Google Scholar] [PubMed]
- 5. Trémollieres FA, Pouillès JM, Drewniak N, Laparra J, Ribot CA, Dargent-Molina P. Fracture risk prediction using BMD and clinical risk factors in early postmenopausal women: Sensitivity of the WHO FRAX tool. J Bone Miner Res 2010;25:1002-9. [Google Scholar] [PubMed]
- 6. Clynes MA, Westbury LD, Dennison EM, Kanis JA, Javaid MK, Harvey NC, et al. Bone densitometry worldwide: A global survey by the ISCD and IOF. Osteoporos Int 2020;31:1779-86. [Google Scholar] [PubMed]
- 7. Didier H, Sanford B. Quantitative ultrasound (QUS) in the management of osteoporosis and assessment of fracture risk. J Clin Densitom 2017;20:322-33. [Google Scholar] [PubMed]
- 8. Oo WM, Naganathan V, Bo MT, Hunter DJ. Clinical utilities of quantitative ultrasound in osteoporosis associated with inflammatory rheumatic diseases. Quant Imaging Med Surg 2018;8:100-13. [Google Scholar] [PubMed]
- 9. Chanprasertpinyo W, Punsawad C, Khwanchuea R, Sukkriang N, Yincharoen P, Rerkswattavorn C. Comparison between calcaneus quantitative ultrasound and the gold standard DXA in the ability to detect osteoporosis in chronic obstructive pulmonary disease patients. J Orthop Surg Res 2023;18:778. Erratum in: J Orthop Surg Res 2023;18:868. [Google Scholar] [PubMed]
- 10. Pluijm SM, Graafmans WC, Bouter LM, Lips P. Ultrasound measurements for the prediction of osteoporotic fractures in elderly people. Osteoporos Int 1999;9:550-6. [Google Scholar] [PubMed]
- 11. Luo W, Wu J, Chen Z, Guo P, Zhang Q, Lei B, et al. Evaluation of fragility fracture risk using deep learning based on ultrasound radio frequency signal. Endocrine 2024;86:800-12. [Google Scholar] [PubMed]
- 12. Natukunda E, Szubert AJ, Bamford A, Doerholt K, Gibb DM, Wandera C, et al. Comparison between dual energy X-ray absorptiometry and calcaneal quantitative ultrasound for determining bone mineral density in children living with HIV in uganda: A cross- sectional study. BMC Pediatr 2025;25:529. [Google Scholar] [PubMed]
- 13. Babhulkar S, Seth S. Prevalence of osteoporosis in India: An observation of 31238 adults. Int J Res Orthop 2021;7:362-8. [Google Scholar] [PubMed]
- 14. Kaushal N, Vohora D, Jalali RK, Jha S. Prevalence of osteoporosis and osteopenia in an apparently healthy Indian population – a cross-sectional retrospective study. Osteoporos Sarcopenia 2018;4:53-60. [Google Scholar] [PubMed]
- 15. Sharma A, Garg A, Singh B. The prevalence of osteopenia and osteoporosis in out-patients above 40 years of a tertiary care hospital in Delhi. Int J Res Orthop 2022;8:222-6. [Google Scholar] [PubMed]
- 16. Khinda R, Valecha S, Kumar N, Walia JP, Singh K, Sethi S, et al. Prevalence and predictors of osteoporosis and osteopenia in postmenopausal women of Punjab, India. Int J Environ Res Public Health 2022;19:2999. [Google Scholar] [PubMed]
- 17. Liu DH, Lin CS, Wu PC. Osteoporosis self-assessment tool for Asians and calcaneal quantitative ultrasound for identifying primary osteoporosis in Taiwanese postmenopausal women. Front Endocrinol (Lausanne) 2025;16:1639176. [Google Scholar] [PubMed]
- 18. Hans D, Durosier C, Kanis JA, Johansson H, Schott-Pethelaz AM, Krieg MA. Assessment of the 10-year probability of osteoporotic hip fracture combining clinical risk factors and heel bone ultrasound: The EPISEM prospective cohort of 12,958 elderly women. J Bone Miner Res 2008;23:1045-51. [Google Scholar] [PubMed]