The subluxated atlas may lead to NPH-like symptoms. NPH-like symptoms were improved by indirect spinal decompression with atlantoaxial reduction and fusion.
Dr. Masaki Tatsumura, Department of Orthopaedic Surgery and Sports Medicine, Tsukuba University Hospital Mito Clinical Education and Training Center, Mito Kyodo General Hospital Mito, 3-2-7 Miyamachi, Mito, Ibaraki 310-0015, Japan. E-mail: tatsumura@md.tsukuba.ac.jp
Introduction: Normal pressure hydrocephalus (NPH) has three characteristics: Gait disorder, urinary incontinence, and amnesia. We report a case of atlantoaxial subluxation-related NPH in a patient whose symptoms were dramatically improved by atlantoaxial fusion surgery.
Case Report: A 67-year-old woman presented complaining of walking disturbance. 4 weeks after her first presentation and initial diagnosis of atlantoaxial subluxation associated with rheumatoid arthritis, the patient fell and fractured her right patella. After open surgery for the fracture with spinal anesthesia, she had urinary incontinence, cognitive decline, and gait disturbance, and brain computed tomography showed enlarged ventricles. Atlantoaxial fusion surgery was performed 8 weeks after the first presentation. Postsurgically, improvement of her memory and urinary function was noted and she was able to walk with the assistance of a cane and perform activities of daily living independently. Even 6 years after the surgery, the enlargement of the ventricles ameliorated, there was no abnormality in cognition, and she was able to walk alone and run a business.
Conclusion: The atlas was subluxated, even when the cervical spine was in a neutral position, and the spinal cord was seriously compressed, which impeded cerebrospinal fluid flow. We speculate changes in cerebrospinal pressure due to spinal anesthesia-induced NPH-like symptoms, which were improved by indirect spinal decompression by atlantoaxial reduction with atlantoaxial fusion.
Keywords: Atlanto-axial fusion, atlantoaxial subluxation, mechanical obstruction of cerebrospinal fluid, normal pressure hydrocephalus, rheumatoid arthritis, spinal analgesia, spinal canal stenosis.
Normal pressure hydrocephalus (NPH) has three characteristics: Gait disorder, urinary incontinence, and amnesia. Among the known etiologies of NPH, mechanical obstruction of cerebrospinal fluid (CSF) flow is important and can be treated by removing the obstruction. Some craniocervical disease such as Arnold–Chiari malformation has been reported to cause NPH [1]. The flow of cerebrospinal fluid is impeded due to physical stenosis in cervical spondylotic myelopathy [2], and posterior cervical decompression improves the CSF return in the cervical spine and normalizes the flow [3]. Although external compression of the dura mater by osseous structures can cause NPH theoretically, there are few previous reports describing NPH caused by bony compression [4] or atlantoaxial subluxation [5,6]. Here, we report a rare case of atlantoaxial subluxation-related NPH in a patient whose symptoms were dramatically improved by atlantoaxial fusion surgery.
A 67-year-old woman presented at our hospital complaining of progressing gait disorder and occipital headache 1 month before her initial visit. She had 14-year history of treatment for rheumatoid arthritis and underwent arthroplasty of the wrist bilaterally at age 55 years. Physical examination at the first visit showed full muscle strength, bilateral hyperreflexia of lower extremities, bilateral upper/lower extremity sensory disturbance, and no vesicorectal dysfunction. The patient showed positive Romberg and Mann test results and impossible tandem gait. Grip strength could be not measured because of deformity of fingers from rheumatoid arthritis.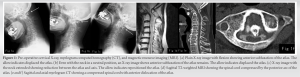
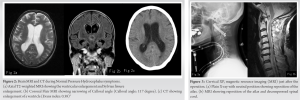
Lateral X-ray images showed anterior subluxation of the atlas in the neutral and flexion position of the neck that was reduced when the neck was extended (Fig. 1a, b, c). In MRI and myelogram CT, spinal cord was seen severely compressed from the anteriorly displaced posterior arch of the atlas even in a neutral position (Fig. 1d, e, f). Surgery was scheduled under the diagnosis of atlantoaxial subluxation. 4 weeks after her initial presentation while awaiting surgery, she fell and fractured her left patella. Therefore, emergency open reduction surgery for patella fracture was performed under lumbar analgesia. Several days after the surgery, the patient complained of difficulty on walking due to strong dizziness. She presented with urinary incontinence and memory disorder. Revised Hasegawa Dementia Scale (HDS-R) at that time was 13 points, indicating severe dementia, and brain CT/MRI showed enlargement of a ventricle (Evans index: 0.38), expansion of a Sylvian fissure, and narrowing of Callosal angle (Callosal angle: 117°), which suggested NPH (Fig. 2a, b, c). CSF findings were in the normal range, the specific gravity was 1.005, pH was 7.953, sugar concentration was 64 mg/dL, and protein concentration was 46 mg/dL. CSF pressure was 80mmH2O.
Atlantoaxial fusion was performed 8 weeks after her initial presentation. The atlas and axis were fixed with bilateral transarticular screws after reducing the atlas, followed by an autologous iliac bone graft (Fig. 3a, b).
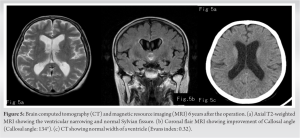
Four weeks after the surgery, clinical symptoms ameliorated, her HDS-R increased to 27 points indicating almost normal, gait improved such that the patient walk with the assistance of a cane, and urinary incontinence disappeared. She became independent and was discharged. Post-operative follow-up brain CT showed attenuation of NPH, of which the Evans index improved to 0.32, fusion of the posterior arch of atlas and lamina of the axis, and attenuation of spinal cord compression (Fig. 4a, b, c, d). Six years later, the patient has no cognitive abnormalities, is able to walk unassisted, and runs a business. Images show no recurrence of NPH at last follow-up (Fig. 5a, b, c).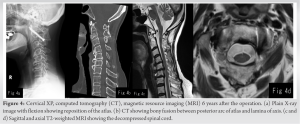
Based on the clinical course and imaging findings, we consider that this patient presented with NPH, which was attenuated by the atlantoaxial fixation. Therefore, NPH in the present case was caused by external compression of dura mater by atlantoaxial subluxation.
We considered the following three hypotheses as the causes of NPH. The first is mechanical compression, the second is increasing CSF viscosity, and the third is increasing CSF supply due to inflammation. A mechanical compression theory has been proposed in which a tumor strongly compressing the spinal cord can cause NPH because it blocks the flow of CSF [7]. A tumor in the thoracolumbar spine can increase CSF pressure [8] and it may cause NPH. A cat model of cervical spinal canal narrowing can produce NPH reproducibly [9]. Most spinal cord tumors reported to cause NPH are located in the lumbar spine, whereas there are only a few reports of cervical spine tumors associated with NPH [10]. A specific feature of the present case was that NPH was caused by obstruction of CSF circulation because of the severe compressed dural tube even with the neck in a neutral position. In addition, the present patient developed NPH after spinal analgesia. After spinal analgesia, cerebrospinal pressure can be increased by injecting a solution of analgesics into the subarachnoid space and cerebrospinal pressure can decrease because of leakage of CSF into the epidural space after puncture. Compliance of the spinal compartment is dependent on increased dura elasticity, epidural space, and atmospheric pressure. The internal pressures in the skull and spinal canal are in equilibrium, and rapid changes in cerebrospinal pressure can be buffered by the spinal subarachnoid space [11]. However, changes in CSF pressure cannot be buffered if the CSF flow is obstructed by a stenosis. In the present case, lumbar puncture may have caused imbalance of the CSF pressure between the skull and the lumbar region due to severe compression of the spinal cord. A tapping test is used as a definitive diagnostic criterion for NPH. However, when there is an obstruction between the brain and lumbar spine so CSF pressure does not rebound rapidly, CSF pressure may not decrease at the brain if the tapping test is performed at the lumbar spine. Supporting this hypothesis is a report that a tapping test shows a false negative in NPH with spinal stenosis [4]. We suspect decompression of intracranial pressure does not occur in some cases with spinal canal stenosis, which may miss NPH and cause misdiagnosis.
Another possible etiology for NPH is inflammation of rheumatoid arthritis. Normal pressure rheumatic hydrocephalus is not uncommon [12]. Prednisolone is reported as effective treatment of NPH with rheumatoid arthritis [5]. Catananti et al. reported that an extra-articular manifestation of rheumatoid arthritis by pathogenic inflammation may cause NPH [6]. In the present case, the symptoms improved after atlantoaxial fusion surgery although we did not change the medication for rheumatoid arthritis, suggesting that the inflammation of rheumatoid arthritis had no substantial impact on development of NPH.
High protein concentration in CSF increases viscosity and may cause NPH [13]. The protein concentration of CSF in the present case was 46 mg/dL, which was within the normal range, and was considered as irrelevant. There is a report of NPH with atlantoaxial subluxation caused by rheumatoid arthritis treated the subluxation with ventriculocardial drainage [14]. Soon et al. reported a similar case with an odontoid pseudotumor related to rheumatoid arthritis, which caused NPH by impaired perfusion and was successfully treated with a ventriculoperitoneal shunt [15]. In our present patient, we speculated that NPH was caused by changes in CSF pressure due to lumbar analgesia in addition to impaired CSF perfusion due to severe canal stenosis. In this case of atlantoaxial subluxation with NPH, both pyramidal symptoms and NPH symptoms could be ameliorated by decompressing the spinal cord and release of CSF flow obstruction by reducing the displaced atlantoaxial spine without shunt surgery.
We report a rare case of NPH caused by atlantoaxial subluxation in a patient whose symptoms were attenuated completely by atlantoaxial fusion surgery. We recommend physicians be aware that NPH may be caused by impaired CSF flow due to external spinal cord compression by severe canal stenosis.
NPH-like symptoms were improved by indirect spinal decompression with atlantoaxial reduction and fusion.
References
- 1.Shakhnovich VA, Mitrofanova EV, Shimanskiy VN, Konovalov NA, Shkarubo AN. Cerebrovenous orthostatic reactivity in pathology of the craniovertebral junction (Chiari malformation). Zh Vopr Neirokhir Im N N Burdenko 2015;79:61-70. [Google Scholar | PubMed]
- 2.Bae YJ, Lee JW, Lee E, Yeom JS, Kim KJ, Kang HS. Cervical compressive myelopathy: Flow analysis of cerebrospinal fluid using phase-contrast magnetic resonance imaging. Eur Spine J 2017;26:40-8. [Google Scholar | PubMed]
- 3.Tominaga T, Watabe N, Takahashi T, Shimizu H, Yoshimoto T. Quantitative assessment of surgical decompression of the cervical spine with cine phase contrast magnetic resonance imaging. Neurosurgery 2002;50:791-5. [Google Scholar | PubMed]
- 4.Komotar RJ, Zacharia BE, Mocco J, Kaiser MG, Frucht SJ, McKhann GM 2nd. Cervical spine disease may result in a negative lumbar spinal drainage trial in normal pressure hydrocephalus: Case report. Neurosurgery 2008;63:315. [Google Scholar | PubMed]
- 5.Markusse HM, Hilkens PH, Van den Bent MJ, Vecht CJ. Normal pressure hydrocephalus associated with rheumatoid arthritis responding to prednisone. J Rheumatol 1995;22:342-3. [Google Scholar | PubMed]
- 6.Catananti C, Mastropaolo S, Calabrese C, Silveri MC, Onder G. A case of normal-pressure hydrocephalus associated with rheumatoid arthritis. Aging Clin Exp Res 2010;22:189-91. [Google Scholar | PubMed]
- 7.Morandi X, Amlashi SF, Riffaud L. A dynamic theory for hydrocephalus revealing benign intraspinal tumours: Tumoural obstruction of the spinal subarachnoid space reduces total CSF compartment compliance. Med Hypotheses 2006;67:79-81. [Google Scholar | PubMed]
- 8.Glasauer FE. Thoracic and lumbar intraspinal tumors associated with increased intracranial pressure. J Neurol Neurosurg Psychiatry 1964;27:451-8. [Google Scholar | PubMed]
- 9.Klarica M, Jukić T, Miše B, Kudelić N, Radoš M, Orešković D. Experimental spinal stenosis in cats: New insight in mechanisms of hydrocephalus development. Brain Pathol 2016;26:701-12. [Google Scholar | PubMed]
- 10.Porter A, Lyons MK, Wingerchuk DM, Bosch EP. Spinal cord astrocytoma presenting as “idiopathic” intracranial hypertension. Clin Neurol Neurosurg 2006;108:787-9. [Google Scholar | PubMed]
- 11.Martins AN, Wiley JK, Myers PW. Dynamics of the cerebrospinal fluid and the spinal dura mater. J Neurol Neurosurg Psychiatry 1972;35:468-73. [Google Scholar | PubMed]
- 12.Ma B, Wu H, Yin H, Chang J, Wang L, Wang R, et al. Management of hydrocephalus associated with autoimmune diseases: A series of 19 cases. Autoimmunity 2017;50:422-7. [Google Scholar | PubMed]
- 13.Gardner WJ, Spitler DK, Whitten C. Increased intracranial pressure caused by increased protein content in the cerebrospinal fluid. N Engl J Med 1954;250:932-6. [Google Scholar | PubMed]
- 14.Collée G, Breedveld FC, Algra PR, Padberg GW. Rheumatoid arthritis with vertical atlantoaxial subluxation complicated by hydrocephalus. Br J Rheumatol 1987;26:56-8. [Google Scholar | PubMed]
- 15.Soon WC, Thanabalasundaram G, Thant KZ, Ogbonnaya ES, Harrisson SE. Obstructive hydrocephalus secondary to odontoid pannus: Case report and review of literature. J Surg Case Rep 2018;2018:1-3. [Google Scholar | PubMed]