The non-vascularized fibular graft can be used as an effective reconstruction tool in the management of bone voids arising in the management of giant aneurysmal bone cysts in long bones.
Dr. Sathish Muthu, Orthopaedic Research Group, Coimbatore, Tamil Nadu, India. E-mail: drsathishmuthu@gmail.com
Introduction: Aneurysmal bone cyst (ABC) is a benign expansile osteolytic lesion, characterized by a blood-filled cavity in the bone. Giant ABC (GABC) is an uncommon condition due to the delayed presentation of an ABC that is difficult to handle when it occurs in long weight-bearing bones due to its aggressive nature. The common treatment relies on total resection of tumor with reconstruction of the resultant defect.
Case Report: We present the results of 5 cases of GABCs of long bones managed with non-vascularized fibular graft in a cross-beam fashion along with internal fixation. All patients achieved complete consolidation of the lesion by 12 months along with full incorporation of the graft with good-excellent musculoskeletal tumor society scores. None of the patients had recurrence/pathological fracture till 2 years of follow-up.
Conclusion: We suggest the method of using a non-vascularized fibula graft in a cross-beam fashion to reconstruct the void from the resection of long-bone GABC as a safe, reliable technique with excellent functional and radiological results.
Keywords: Giant Aneurysmal Bone Cyst, Fibular Graft, Resection, Recurrence, Reconstruction
Aneurysmal bone cysts (ABCs) were first reported by Jaffe and Lichenstein in 1942 [1]. ABCs are uncommon bone lesions comprising 1–2% of primary bone tumors. The tumor predominantly occurs in the first two decades with a mean age of presentation around 18 years [2]. The ABC is a lesion of the pseudotumor type, and it can be defined as a cavity filled with blood that has septations made of connective tissue. The mechanism of formation of these tumors is considered to be sudden venous or arteriovenous malformation [3,4]. Pathologically, they contain fusiform cells, multinucleated giant cells, hemosiderin storage areas, and some trabecular bone [5]. The giant ABCs (GABCs) are late presenting ABCs that are very destructive and expansile forms of ABCs which will produce more chance of pathological fracture and disability [6]. They tend to be located eccentrically in the metaphysis of long bones adjacent to the physis region. Although ABCs are benign lesions, there are cases reported of their malignant transformation [7]. They are considered locally aggressive and possess a higher rate of recurrence [1,8]. The predominant distribution remains to lower limbs (50–60%) of which the tibia (40%), especially the proximal tibia (24%), followed by fibula (20%) [5,9,10]. The clinical feature usually presents with pain, mass, swelling, pathologic fracture, or a combination of these symptoms in the affected area. The symptoms are usually present for several weeks to months before the diagnosis is made, and the patient may also have a history of a rapidly enlarging mass. Neurologic symptoms associated with GABCs may develop secondary to pressure or tenting of the nerve over the lesion, typically in the spine [3,7,10]. Pathologic fracture occurs in about 8% of ABCs, but the incidence may be as high as 21% in ABCs that have spinal involvement [5,11]. The ABCs are commonly managed with extended curettage, intralesional sclerotherapy injection, intralesional-doxycycline injection, systemic bisphosphonates, denosumab, or selective arterial embolization in areas where surgical exposure is difficult [12-14]. In contrast, GABCs of long bone need total resection of tumor with the reconstruction of the defect using bone graft with or without internal fixation depending on the size and site of the lesion. Among the various cancellous and cortical bone grafts, the non-vascularized fibula, a strong, long, tubular bone, can be used as a strut graft in place of a large defective cavity not only to fill the void but also for additional mechanical stability so that patient can be mobilized as early as visible consolidation of the graft with the native bone [15-17]. Previous reports on the use of non-vascularized fibula for the reconstruction of ABCs have been shown to give radiologically and functionally appealing results [6,18,19]. However, literature on the use of non-vascularized fibula for reconstruction in the management of GABCs remains limited. Hence, the main aim of this article is to present the role of non-vascularized fibula in the management of GABC in long bones of limbs in a particular cross-beam fashion taking advantage of its mechanical strength.
We report the results of 5 patients (M:F=3:2) who were diagnosed with GABCs in the long bones and managed with extended curettage and reconstruction with non-vascularized fibular graft supplemented with buttress plating. The age group of patients ranged between 17 and 44 years. The size of the lesion ranged from 18 cm2 to 72 cm2. The lesions were more common in the long bones of the lower limb as discussed earlier with preponderance for the proximal tibia (n = 2) and proximal femur (n = 2) while one case was noted in the proximal humerus. All the lesions were insidious in onset and slow growing with duration ranging from 4 months to 1 year before presentation. All the lesions were biopsied and found to be of Grade II (n = 3) and Grade III (n = 2) as per Enneking’s grading of the lesion. All the patients were pre-operatively evaluated for the functional status with musculoskeletal tumor society (MSTS) score. The detailed clinical characteristics of the cases are presented in Table 1.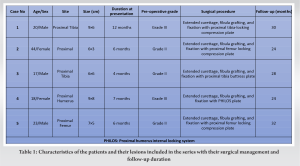
Surgical technique
The lesion is approached following the general principles of tumor dissection and the entire lesion is debrided with extended curettage using phenol and electrical cauterization of the bleeding walls. The cavity is further washed with hydrogen peroxide for chemical cauterization. Usually, the fibula is harvested from the ipsilateral side. An 8–10 cm section of the middle third of the fibula is harvested subperiosteally following the posterolateral approach, leaving 5 cm of the proximal fibula and 8 cm of the distal segment to avoid joint instability. The debrided cavity is supported with the fibular strut in a cross-beam fashion as illustrated in Fig. 1 to prevent the collapse of the cavity walls and aid in maintaining the space for faster consolidation and weight bearing. The remainder of the cavity is filled with cancellous grafts harvested from the contralateral iliac crest regularly. No cement or artificial bone substitutes were utilized to fill the cavity. Further, the construct is augmented with a buttress plate and screws to prevent further collapse of the subchondral bone till the fibular strut graft incorporates with the native bone. The curretted samples were sent to histopathology for secondary staging and confirmation of the diagnosis. All the lesions were confirmed to be ABCs with cyst walls made up of fibroblasts, woven bone, and osteoclastic giant cells. All patients were immobilized with appropriate methods in the immediate post-operative period. Isometric exercises were initiated in 1st week, mobilization exercises in 2nd week, and strengthening exercises in the 3rd week. From 3rd-week onward, non-weight-bearing walking was started with walker support. Patients were maintained on non-weight bearing until consolidation of the lesion became radiologically evident which occurred by a mean duration of 17 weeks (range 14–19 weeks) following which partial and full weight bearing was gradually resumed as tolerated.
Further, the construct is augmented with a buttress plate and screws to prevent further collapse of the subchondral bone till the fibular strut graft incorporates with the native bone. The curretted samples were sent to histopathology for secondary staging and confirmation of the diagnosis. All the lesions were confirmed to be ABCs with cyst walls made up of fibroblasts, woven bone, and osteoclastic giant cells. All patients were immobilized with appropriate methods in the immediate post-operative period. Isometric exercises were initiated in 1st week, mobilization exercises in 2nd week, and strengthening exercises in the 3rd week. From 3rd-week onward, non-weight-bearing walking was started with walker support. Patients were maintained on non-weight bearing until consolidation of the lesion became radiologically evident which occurred by a mean duration of 17 weeks (range 14–19 weeks) following which partial and full weight bearing was gradually resumed as tolerated.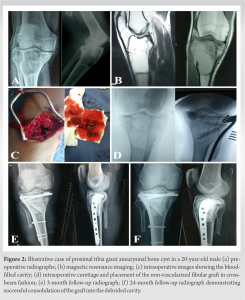 Patients were serially followed up with functional scoring with MSTS score and radiological evaluation every 3 months for 1 year and every 6 months thereafter. The scores improved significantly from the pre-operative mean score of 2.2 (poor grade) to 23.6 (excellent grade) at 2-year follow-up as shown in Table 2.
Patients were serially followed up with functional scoring with MSTS score and radiological evaluation every 3 months for 1 year and every 6 months thereafter. The scores improved significantly from the pre-operative mean score of 2.2 (poor grade) to 23.6 (excellent grade) at 2-year follow-up as shown in Table 2. It is also noted from Table 2 that the post-operative scores do not depend on the age, sex, location, or size of the lesion among the included patients. None of the included patients reported any complications, donor site morbidity, or recurrence at 2-year follow-up. The additional appropriate internal fixation gave good mechanical stability resulting in complete consolidation of the lesion within 12 months. Fibular grafts were fully incorporated by mean duration of 13.8 (range 12–15) months.
It is also noted from Table 2 that the post-operative scores do not depend on the age, sex, location, or size of the lesion among the included patients. None of the included patients reported any complications, donor site morbidity, or recurrence at 2-year follow-up. The additional appropriate internal fixation gave good mechanical stability resulting in complete consolidation of the lesion within 12 months. Fibular grafts were fully incorporated by mean duration of 13.8 (range 12–15) months.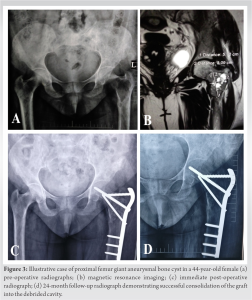 All patients went back to their daily activities by mean duration of 10.2 (range 9–12) months. Figs. 2 and 3 show illustrative case examples in the proximal tibia and proximal femur, respectively.
All patients went back to their daily activities by mean duration of 10.2 (range 9–12) months. Figs. 2 and 3 show illustrative case examples in the proximal tibia and proximal femur, respectively.
ABCs are benign lesions with a <1% chance of malignant transformation [5]. While the majority of these tumors are primary bone lesions, 30% are secondary to certain other bone lesions such as giant cell tumors, chondroblastoma, eosinophilic granuloma, browns tumors, chondromyxoid fibroma, non-ossifying fibroma, and telangiectatic osteosarcoma [20,21]. Overall, GABCs are the late presenting ABCs making them presented in large sizes with aggressive local expansions mimicking malignant lesions [6]. Radiologically, they are found to be sharp expansile lesions with thin sclerotic margins with evident fluid levels [22]. The management of ABCs depends on the time of diagnosis. In 25% of cases, lack of early radiological or medical diagnosis gives management difficulty in these types of tumors [12,13]. This delayed presentation often makes the tumor locally aggressive massive lesions presenting with mechanical symptoms to be labeled as GABCs [6]. In small ABCs, the routine management ranges from minimally invasive curettage and bone grafting which may be allogenic or autologous depending on the size of the lesion. These tumors have a high rate of local recurrence with routine curettage ranging from 18% to 59% [2,23,24]. To prevent the high recurrence, various adjuvants such as a high-speed burr, argon beam coagulation, phenol, liquid nitrogen, cementation, and sclerotherapy have been used to be called extended curettage [25-27]. Alternatively, arterial embolization and radiotherapy have also been used [28]. However, radiotherapy is associated with a risk of malignant transformation [14]. They are usually reserved for lesions in difficult-to-reach areas for surgical management. GABCs that present with large expansible lesions around the metaphysis of long weight-bearing bones possess a high risk of pathological fracture and present with severe pain due to local soft-tissue compression. Often, these cases present with pathological fractures where management not only depends on the excision of the lesion but also needs mechanical strength to provide hassle-free mobility in the future [10]. However, none of the included cases presented with pathological fractures while pain and swelling were their presenting complaints. In these conditions, compared to vascularized fibular graft that demands surgical expertise with significant donor site morbidity, non-vascularized fibular grafts are a good option for reconstruction of the void arising from extended curettage of the lesion [19,25]. Furthermore, vascularized fibular graft needs an extensive approach with increased operating time which may increase the risk of other complications [29]. The geometric orientation of the non-vascularized fibula is of great importance to maintain the anatomic structural integrity of the void developed from the curettage. The authors consider the cross-beam concept of graft placement bridging the diagonal ends of the cavity with the cortical strut of the fibula to prevent further collapse and early incorporation with the native bone. The rest of the dead space can be filled with either iliac crest autograft or cancellous femoral head allograft depending on the availability. Apart from the fibula, other void management strategies such as the sandwich technique of using cement and autologous bone grafts, complete cement filing, and the usage of artificial bone substitutes and tissue engineering techniques are also in vogue [30-32]. They have other limitations such as cost, delayed bone ingrowth, and reduced osteogenic potential compared to the current technique described. The basic rationale in the management of GABCs involves the preservation of the length of the long bone. Hence, we used fibular graft along with cancellous iliac crest autograft to improve better uptake of the non-vascularized fibular graft. The cortical nature of fibular graft in cross-beam architecture provides immediate stability and maintains the length necessary. The implanted graft will aid in the osteogenic induction with the help of the overlying periosteum and surrounding soft tissues where in our cases two involve the proximal tibia, two proximal femurs, and one proximal humerus. All these regions have a good local blood supply and along with autologous iliac crest grafts add to the osteogenic potential to form a favorable environment for new bone formation and incorporation of the fibular graft and reconstitution of the bone void. Although the malignant transformation potential of the ABCs remains limited, the particular malignant transformation potential in GABCs remains unknown [5]. We did not note any recurrence or malignant transformation on histopathological examination in our series. However, long-term follow-up of these lesions is needed to account for their recurrence rate and risk of malignant transformation.
GABCs should be diagnosed and managed with extra care. Non-vascularized fibular graft with iliac crest autograft remains a safe and satisfactory method in GABC management with excellent functional and radiological results. Long-term follow-up studies are required to provide information regarding the recurrence and risk of malignant transformation in these GABCs.
- GABCs are late presenting ABCs with pressure symptoms as their presenting complaint.
- The cross-beam architecture of a non-vascularized fibula graft aids in maintaining the length of the void developed from extended curettage.
- Non-vascularized fibular graft with iliac crest autograft remains a safe and satisfactory method in GABC management with excellent functional and radiological results.
- Long-term follow-up studies are required for early detection of recurrence due to their aggressive nature.
References
- 1.Jaffe HL, Lichtenstein L. Solitary unicameral bone cyst: With emphasis on the roentgen picture, the pathologic appearance and the pathogenesis. Arch Surg 1942;44:1004-25. [Google Scholar | PubMed]
- 2.Mankin HJ, Hornicek FJ, Ortiz-Cruz E, Villafuerte J, Gebhardt MC. Aneurysmal bone cyst: A review of 150 patients. J Clin Oncol 2005;23:6756-62. [Google Scholar | PubMed]
- 3.Mendenhall WM, Zlotecki RA, Gibbs CP, Reith JD, Scarborough MT, Mendenhall NP. Aneurysmal bone cyst. Am J Clin Oncol 2006;29:311-5. [Google Scholar | PubMed]
- 4.Leithner A, Windhager R, Lang S, Haas OA, Kainberger F, Kotz R. Aneurysmal bone cyst. A population based epidemiologic study and literature review. Clin Orthop Relat Res 1999;363:176-9. [Google Scholar | PubMed]
- 5.Stevens KJ, Stevens JA. Aneurysmal bone cysts. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2023. [Google Scholar | PubMed]
- 6.Abuhassan FO, Shannak A. Non-vascularized fibular graft reconstruction after resection of giant aneurysmal bone cyst (ABC). Strategies Trauma Limb Reconstr 2010;5:149-54. [Google Scholar | PubMed]
- 7.Brindley GW, Greene JF, Frankel LS. Case reports: Malignant transformation of aneurysmal bone cysts. Clin Orthop Relat Res 2005;438:282-7. [Google Scholar | PubMed]
- 8.Schajowicz F, Sissons HA, Sobin LH. The World Health Organization’s histologic classification of bone tumors. A commentary on the second edition. Cancer 1995;75:1208-14. [Google Scholar | PubMed]
- 9.Kaplanoğlu V, Ciliz DS, Kaplanoğlu H, Elverici E. Aneurysmal bone cyst of the calcaneus. J Clin Imag Sci 2014;4:60. [Google Scholar | PubMed]
- 10.Babazadeh S, Broadhead ML, Schlicht SM, Powell GJ, Tymms GM. Pathologic fracture of a calcaneal aneurysmal bone cyst. J Foot Ankle Surg 2011;50:727-32. [Google Scholar | PubMed]
- 11.Kitamura T, Ikuta K, Senba H, Komiya N, Shidahara S. Long-term follow-up of a case of aneurysmal bone cyst in the lumbar spine. Spine J 2013;13:e55-8. [Google Scholar | PubMed]
- 12.Deventer N, Deventer N, Gosheger G, de Vaal M, Vogt B, Budny T. Current strategies for the treatment of solitary and aneurysmal bone cysts: A review of the literature. J Bone Oncol 2021;30:100384. [Google Scholar | PubMed]
- 13.Mohaidat ZM, Al-Gharaibeh SR, Aljararhih ON, Nusairat MT, Al-Omari AA. Challenges in the diagnosis and treatment of aneurysmal bone cyst in patients with unusual features. Adv Orthop 2019; 2019:2905671. [Google Scholar | PubMed]
- 14.Elsayad K, Kriz J, Seegenschmiedt H, Imhoff D, Heyd R, Eich HT, et al. Radiotherapy for aneurysmal bone cysts : A rare indication. Strahlenther Onkol 2017;193:332-40. [Google Scholar | PubMed]
- 15.Enneking WF, Eady JL, Burchardt H. Autogenous cortical bone grafts in the reconstruction of segmental skeletal defects. J Bone Joint Surg Am 1980;62:1039-58. [Google Scholar | PubMed]
- 16.Wilson PD. A clinical study of the biomechanical behavior of massive bone transplants used to reconstruct large bone defects. Clin Orthop Relat Res 1972;87:81-109. [Google Scholar | PubMed]
- 17.Sailesh SS, Muthu S, Ismail ND. Hemi-fibular grafting for metacarpal giant cell tumor-surgical technique. J Orthop Case Rep 2020;10:80-4. [Google Scholar | PubMed]
- 18.Ansari MT, Gautam D, Kotwal PP. Mother’s fibula in son’s forearm: Use of maternal bone grafting for aneurysmal bone cyst not amenable to curettage-a case report with review of literature. SICOT-J 2016;2:18. [Google Scholar | PubMed]
- 19.Yadav SS. Dual-fibular grafting for massive bone gaps in the lower extremity. J Bone Joint Surg Am 1990;72:486-94. [Google Scholar | PubMed]
- 20.Hakim DN, Pelly T, Kulendran M, Caris JA. Benign tumours of the bone: A review. J Bone Oncol 2015;4:37-41. [Google Scholar | PubMed]
- 21.Rapp TB, Ward JP, Alaia MJ. Aneurysmal bone cyst. J Am Acad Orthop Surg 2012;20:233-41. [Google Scholar | PubMed]
- 22.Duran S, Kose CC, Guresci S. An extra-articular ganglion cyst with multiple fluid-fluid levels. Curr Med Imaging 2024;20: e080823219538 [Google Scholar | PubMed]
- 23.Park HY, Yang SK, Sheppard WL, Hegde V, Zoller SD, Nelson SD, et al. Current management of aneurysmal bone cysts. Curr Rev Musculoskelet Med 2016;9:435-44. [Google Scholar | PubMed]
- 24.Capanna R, Bettelli G, Biagini R, Ruggieri P, Bertoni F, Campanacci M. Aneurysmal cysts of long bones. Ital J Orthop Traumatol 1985;11:409-17. [Google Scholar | PubMed]
- 25.Rahman MA, El Masry AM, Azmy SI. Review of 16 cases of aneurysmal bone cyst in the proximal femur treated by extended curettage and cryosurgery with recons using autogenous nonvascularized fibula graft. J Orthop Surg (Hong Kong 2018;26(12). [Google Scholar | PubMed]
- 26.Yenigül AE, Sofulu Ö, Erol B. Treatment of locally aggressive benign bone tumors by means of extended intralesional curettage without chemical adjuvants. SAGE Open Med 2022;10:20503121221094199. doi: 10.1177/20503121221094199. [Google Scholar | PubMed | CrossRef]
- 27.Solooki S, Keikha Y, Vosoughi AR. Can ethanol be used as an adjuvant to extended curettage in order to reduce the recurrence rate of aneurysmal bone cyst? Rev Brasil Ortop 2017;52:349-53. [Google Scholar | PubMed]
- 28.Cevolani L, Staals E, Campanacci L, Dozza B, Martella C, Spinnato P, et al. Aneurysmal bone cyst: Is selective arterial embolization effective as curettage and bone grafting? J Surg Oncol 2023;128(8):1428-36. [Google Scholar | PubMed]
- 29.Gorski SM, Dong C, Lenze U, Haug M, Krieg AH. Vascularized and non-vascularized fibula grafts in tumour reconstruction: Single centre experience with mid to long-term results. Anticancer Res 2022;42:5443-7. [Google Scholar | PubMed]
- 30.Liu Q, Long F, Zhang C, Liu Y, He H, Luo W. Biological reconstruction of bone defect after resection of malignant bone tumor by allograft: A single-center retrospective cohort study. World J Surg Oncol 2023;21:234. [Google Scholar | PubMed]
- 31.Migliorini F, La Padula G, Torsiello E, Spiezia F, Oliva F, Maffulli N. Strategies for large bone defect reconstruction after trauma, infections or tumour excision: A comprehensive review of the literature. Eur J Med Res 2021;26:118. [Google Scholar | PubMed]
- 32.Nishida J, Shimamura T. Methods of reconstruction for bone defect after tumor excision: A review of alternatives. Med Sci Monit 2008;14:RA107-13. [Google Scholar | PubMed]









