The combination of Sprengel deformities, mirror movements synkinesis, and arthrogryposis could co-exist and hence should be evaluated when one of them is observed clinically.
Dr. Jojin Jose Chitten, consultant orthopaedic surgeon, Holy Family superspeciality hospital, Muthalakkodam, Kerala, India. E-mail: jojinjosec@gmail.com
Introduction: Bilateral Sprengel deformities, mirror movements synkinesis, and arthrogryposis are described in different combinations in various syndromes but never together.
Case Report: We present a 12-year-old girl who presented with bilateral shoulder deformities and difficulty in coordination while writing. On examination, she was noted to have bilateral Sprengel deformities with flexion contractures of upper-limb joints and mirror movements of both upper and lower-limb joints.
Conclusion: In the light of relevant literature, we may speculate that these three have a causal relation and even a genetic basis but further studies are needed to prove the same.
Keywords: Sprengel deformity, mirror movements, synkinesis, arthrogryposis.
Sprengel deformity is a rare congenital anomaly of the shoulder girdle. It is caused by an interruption of the normal caudal migration of the scapula. Arthrogryposis is a clinical presentation in many syndromes but has never been associated with syndromes associated with Sprengel deformity. Sprengel deformity and arthrogryposis are rare anomalies and the etiopathogenesis of both is still unclear. Both anomalies rarely occur in isolation. There are congenital syndromes with mirror movements and either Sprengel deformity or arthrogryposis, but all three have never been reported together.
A 12-year-old girl presented with non-progressive congenital flexion deformities of bilateral elbows and complaints of difficulty in writing, typing, jogging, and difficulty in using the Indian toilet such that she had to sit with one leg extended for balance. She was the firstborn child of a second-degree consanguineous marriage. Her mother gave a history of delayed and decreased fetal movements during pregnancy. Her motor milestones were delayed though social adaptive and language milestones were normal. She was good at academics. She had attained menarche at the age of 12 years and has normal menstrual cycles. Family history was unremarkable. On clinical examination, she had prominence in the suprascapular region on both sides with trapezii contracture (Fig. 1) and mild-to-moderate restriction of cervical spine range of motion in rotation and lateral bending. She had 20° of internal rotation deformity bilaterally and abduction was restricted to 120° at both the shoulders.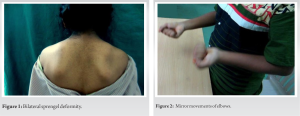 She had 22° of fixed flexion deformity at the left elbow and 20° at the right elbow. Bilateral passive forearm supination was restricted to 60° with passive wrist dorsiflexion restricted to 35° on both sides. Clinodactyly was noticed in bilateral ring fingers and her fingers were hyperextensible at the interphalangeal joints. Otherwise, there were no signs of generalized ligamentous laxity. She had hypoplastic left thenar eminence with MRC Grade III power of the thenar muscles. She had bilateral tendo Achilles contractures with no passive dorsiflexion possible from neutral though the plantar flexion was full. She had a propulsive gait with a restricted arm swing on both sides.
She had 22° of fixed flexion deformity at the left elbow and 20° at the right elbow. Bilateral passive forearm supination was restricted to 60° with passive wrist dorsiflexion restricted to 35° on both sides. Clinodactyly was noticed in bilateral ring fingers and her fingers were hyperextensible at the interphalangeal joints. Otherwise, there were no signs of generalized ligamentous laxity. She had hypoplastic left thenar eminence with MRC Grade III power of the thenar muscles. She had bilateral tendo Achilles contractures with no passive dorsiflexion possible from neutral though the plantar flexion was full. She had a propulsive gait with a restricted arm swing on both sides.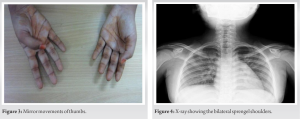 Mirror movements were demonstrated in bilateral fingers, elbows, toes, and ankles. (Fig. 2 & 3) Mirror movements of the toes and fingers worsened with fatigue, but at the elbows and ankles, the mirror movements became less frequent as the voluntary movements were continued. Neurological examination was otherwise normal. Her height for her age was normal. There were no craniofacial malformations, hearing impairment, or visual defects.
Mirror movements were demonstrated in bilateral fingers, elbows, toes, and ankles. (Fig. 2 & 3) Mirror movements of the toes and fingers worsened with fatigue, but at the elbows and ankles, the mirror movements became less frequent as the voluntary movements were continued. Neurological examination was otherwise normal. Her height for her age was normal. There were no craniofacial malformations, hearing impairment, or visual defects.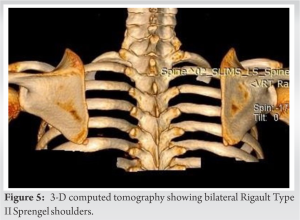
Investigations
X-rays and 3-D computed tomography (CT) scan revealed bilateral Rigault Grade II Sprengel deformities (Figs. 4 & 5) and mild scoliosis at the cervical spine segment, cervicothoracic junction, and thoracolumbar junction (Fig. 6). There was no fusion at any vertebral level. MRI revealed no central nervous system (CNS) or spinal cord malformations. Ultrasonography revealed no renal or genitourinary anomalies. ECHO showed mild aortic regurgitation.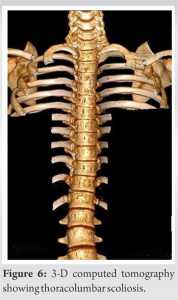
Treatment
She started on regular joint range of motion exercises, muscle stretching and strengthening physiotherapy, and retraining therapy with techniques for increasing wanted movements while focusing on restricting unwanted movements.
Outcome and follow-up
Her frequency of mirror movements decreased and writing and typing co-ordination was getting better. She is compliant with therapy and on regular follow-up.
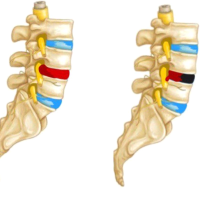
The scapula usually descends to its thoracic location at about 8–12 weeks of gestation, having formed from the paraxial mesoderm at about the level of the fourth or fifth cervical vertebrae [1]. Failure of this descent leads to Sprengel deformity. The deformity is usually unilateral. In unilateral cases, the deformity is easily detected because of asymmetry, unlike in bilateral deformities where symmetry is usually maintained and is cosmetically more acceptable [2]. The diagnosis is confirmed by X-ray or CT scans, showing a high scapula. The Rigault classification is used to grade these deformities. In Grade I deformity, the superomedial angle of the scapula is lower than T2 but above T4 transverse process. In Grade II deformity, the superomedial angle of the scapula is located between the C5 and T2 transverse process, and in Grade III, the superomedial angle of the scapula is above the C5 transverse process. The development of bone, cartilage, and muscles is also affected, resulting in dysplasia and malposition of the scapula. The trapezii and spino-scapular muscles may be fibrotic and contracted. The pectoralis major, latissimus dorsi, and sternocleidomastoids may be hypoplastic and similarly affected, causing limitation of neck and shoulder movements [2]. Sprengel deformity has never been reported in association with arthrogryposis multiplex congenita, which is characterized by congenital, non-progressive contractures in more than two joints and multiple body areas. It is usually symmetrical, but, less frequently, various joints may be involved to a different extent [3]. The diagnosis is purely descriptive, and arthrogryposis can be associated with many syndromes [4]. The degree to which muscles and joints are affected may vary from person to person. In some cases, only a few joints may be affected, and the range of motion may be nearly normal, and in severe cases, almost all the joints may be affected, including the jaw and back [3,5]. Compromised fetal mobility is the main factor leading to fibrosis and contractures of the affected joints [4]. The cause can be pathology in the peripheral or CNS (such as neuronal migration abnormalities, pyramidal disorders, and olivopontocerebellar disorders), connective tissue disorders, defects in neuromuscular transmission, the compromised vascular supply to the fetus, single gene changes (autosomal dominant, autosomal recessive, and X-linked), chromosomal abnormalities, and various syndromes. Neurologic abnormalities are the most common cause of arthrogryposis [6]. The neurogenic groups were also characterized by a high incidence of other congenital anomalies, while the myopathic group had few associated defects. According to Cohen et al., mirror movements are defined as involuntary, synkinetic mirror reversals of an intended movement of the opposite side [7]. Deleted colorectal cancer (DCC) gene mutations were reported in two families with congenital mirror movements [8]. DCC is the receptor for netrin, a protein that guides axon migration of developing neurons across the body’s midline. Normal neuronal migration occurs in the third to fifth months of gestation. Congenital synkinesis may be the only physical examination finding that indicates an axonal migration disorder [9]. Congenital syndromes such as Klippel–Feil syndrome, Moebius syndrome, and Gorlin syndrome are known to have an association with axonal migration disorders which may be missed if mirror movements are not specifically looked for [10-12]. Finger and ocular movements require precise motor control, and errors in the innervation of these muscles may be more easily detected than errors in the wiring of larger muscle groups [9]. Exciting advances in neuroimaging and genetics, however, are revolutionizing the ability to define axon guidance disorders, and it is likely that the associated syndromes are only the first of an important new category of such human neurodevelopmental disorders. In our patient, the propulsive gait and difficulty using the Indian toilet were explained by the bilateral tendo Achilles contractures. She also had symmetrical internal rotation contractures at the shoulders, flexion contractures of the elbows, pronation contractures of the forearms, with associated clinodactyly, hyperextensible interphalangeal joints of the hands, and unilateral thenar muscle weakness, all suggesting arthrogryposis. The parents had not noticed the Sprengel deformities since it was bilaterally symmetrical and was not cosmetically disfiguring. The difficulty in writing and typing was demonstrated to be secondary to mirror movement synkinesis, which is a sign of axonal migration disorders. It is a sign that could be missed if not specifically looked for since it is not a part of routine neurological evaluation. In our case, the lack of family history may suggest a sporadic mutation, an incompletely penetrant autosomal dominant disorder, or in view of parental consanguinity, an autosomal recessive pattern of inheritance. Mirror movements, Sprengel deformity, and arthrogryposis are described in different combinations in various syndromes but never together. Arthrogryposis and mirror movement synkinesis are reported with Klippel–Feil syndrome, Moebius syndrome, and Arnold–Chiari malformation [12-16]. Both Sprengel deformity and arthrogryposis have been separately associated with the del22q11.2 in DiGeorge syndrome and velocardiofacial syndrome, respectively [3,4,17,18]. In addition, we now report a novel combination of Sprengel deformity, mirror movements, and arthrogryposis. Furthermore, Sprengel deformity, arthrogryposis, and axonal migration disorders occur almost at the same fetal age [19-22]. Thus, it is possible that they may arise due to a common developmental defect. Bouwes, Bavinck, and Weaver hypothesized that Sprengel deformity, Klippel–Feil syndrome, and Moebius syndrome are the result of interruption of the early embryonic blood supply in the subclavian arteries, the vertebral arteries, and/or their branches [23-25]. However, Matsuoka et al. considered Klippel–Feil syndrome, Sprengel deformity, and Arnold–Chiari malformation to be defects of postotic neural crest cells [1]. As of now, the etiology of these syndromes remains unknown. It is possible that these syndromes may represent a clinical spectrum of disorders arising from a common genetic mutation or developmental defect.
- To the best of our knowledge, the association of Sprengel deformity, arthrogryposis, and mirror movements synkinesis is a novel combination.
- This case report points out the importance of looking for associated Sprengel deformities and mirror synkinesis in the setting of varied forms of arthrogryposis and intervening as necessary.
- In the light of relevant literature, we may speculate that these have a causal relation and even a genetic basis, but further studies are needed to prove the same.
A thorough clinical examination is mandatory in patients presenting with Sprengel deformities to rule out other joint contractures and mirror movement synkinesis so that these can be evaluated and treated early.
References
- 1.Matsuoka T, Ahlberg PE, Kessaris N, Iannarelli P, Dennehy U, Richardson WD, et al. Neural crest origins of the neck and shoulder. Nature 2005; 436:347-55. [Google Scholar | PubMed]
- 2.Thacker MM, Feldman DS. Sprengel Deformity. Medscape 2016; 8:220-22 [Google Scholar | PubMed]
- 3.Bevan WP, Hall JG, Bamshad M, Staheli LT, Jaffe KM, Song K. Arthrogryposis multiplex congenita (amyoplasia): An orthopaedic perspective. J Pediatr Orthop 2007; 27:594-600. [Google Scholar | PubMed]
- 4.Hall JG. Arthrogryposis multiplex congenita: Etiology, genetics, classification, diagnostic approach, and general aspects. J Pediatr Orthop B 1997; 6:159-66. [Google Scholar | PubMed]
- 5.Steinberg B, Nelson VS, Feinberg SE, Calhoun C. Incidence of maxillofacial involvement in arthrogryposis multiplex congenita. J Oral Maxillofac Surg 1996; 54:956-9. [Google Scholar | PubMed]
- 6.Kalampokas E, Kalampokas T, Sofoudis C, Deligeoroglou E, Botsis D. Diagnosing arthrogryposis multiplex congenita: A review. ISRN Obstet Gynecol 2012; 2012:264918. [Google Scholar | PubMed]
- 7.Cohen LG, Meer J, Tarkka I, Bierner S, Leiderman DB, Dubinsky RM, et al. Congenital mirror movements. Abnormal organization of motor pathways in two patients. Brain 1991; 114:381-403. [Google Scholar | PubMed]
- 8.Srour M, Rivière JB, Pham JM, Dubé MP, Girard S, Morin S, et al. Mutations in DCC cause congenital mirror movements. Science 2010; 328:592. [Google Scholar | PubMed]
- 9.Engle EC. Human genetic disorders of axon guidance. Cold Spring Harb Perspect Biol 2010;2: a001784. [Google Scholar | PubMed]
- 10.Galléa C, Popa T, Billot S, Méneret A, Depienne C, Roze E. Congenital mirror movements: A clue to understanding bimanual motor control. J Neurol 2011; 258:1911-9. [Google Scholar | PubMed]
- 11.Sag E, Gocmen R, Yildiz FG, Ozturk Z, Temucin C, Teksam O, et al. Congenital mirror movements in gorlin syndrome: A case report with DTI and functional MRI features. Pediatrics 2016;137:e20151771. [Google Scholar | PubMed]
- 12.Webb BD, Frempong T, Naidich TP, Gaspar H, Jabs EW, Rucker JC. Mirror movements identified in patients with moebius syndrome. Tremor Other Hyperkinet Mov (N Y) 2014; 4:256. [Google Scholar | PubMed]
- 13.Erol FS, Ucler N, Yakar H. The association of Chiari type III malformation and Klippel-Feil syndrome with mirror movement: A case report. Turk Neurosurg 2011; 21:655-8. [Google Scholar | PubMed]
- 14.Schott GD, Wyke MA. Congenital mirror movements. J Neurol Neurosurg Psychiatry 1981; 44:586-99. [Google Scholar | PubMed]
- 15.Kaissi AA, Kalchauser G, Grill F, Klaushofer K. Arthrogryposis multiplex congenital in a child manifesting phenotypic features resembling dysosteosclerosis/osteosclerosis malformation complex; 3DCT scan analysis of the skull base. Cases J 2008; 1:56. [Google Scholar | PubMed]
- 16.Krishnakumar P, Jisha P. Congenital mirror movements in a child with Chiari type 1 malformation. Indian Pediatr 2007; 44:152-3. [Google Scholar | PubMed]
- 17.Devriendt K, Swillen A, Gewillig M, Fryns JP, Moens P, De Smet L. Velocardiofacial syndrome presenting as distal arthrogryposis. Eur J Pediatr 2004; 163:329-30. [Google Scholar | PubMed]
- 18.Radio FC, Digilio MC, Capolino R, Dentici ML, Unolt M, Alesi V, et al. Sprengel anomaly in deletion 22q11.2 (DiGeorge/Velo-cardio-facial) syndrome. Am J Med Genet A 2016; 170:661-4. [Google Scholar | PubMed]
- 19.Bindoudi A, Kariki EP, Vasiliadis K, Tsitouridis I. The rare sprengel deformity: Our experience with three cases. J Clin Imaging Sci 2014; 4:55. [Google Scholar | PubMed]
- 20.Hyett J, Noble P, Sebire NJ, Snijders R, Nicolaides KH. Lethal congenital arthrogryposis presents with increased nuchal translucency at 10-14 weeks of gestation. Ultrasound Obstet Gynecol 1997; 9:310-3. . [Google Scholar | PubMed]
- 21.Madazli R, Tüysüz B, Aksoy F, Barbaros M, Uludağ S, Ocak V. Prenatal diagnosis of arthrogryposis multiplex congenita with increased nuchal translucency but without any underlying fetal neurogenic or myogenic pathology. Fetal Diagn Ther 2002; 17:29-33. [Google Scholar | PubMed]
- 22.Gressens P. Mechanisms and disturbances of neuronal migration. Pediatr Res 2000; 48:725-30. [Google Scholar | PubMed]
- 23.Ogden JA, Conlogue GJ, Phillips MS, Bronson ML. Sprengel’s deformity. Radiology of the pathologic deformation. Skeletal Radiol 1979; 4:204-11. [Google Scholar | PubMed]
- 24.Engel D. The etiology of the undescended scapula and related syndromes. J Bone Joint Surg 1943; 25:613-25. [Google Scholar | PubMed]
- 25.Oxnard CE. Evolution of the human shoulder: Some possible pathways. Am J Phys Anthropol 1969; 30:319-31. [Google Scholar | PubMed]