Yadav–Kunal reflex can be used to clinically assess the spasticity and recovery following surgery in upper motor neuron lesions.
Dr. Kishor Kunal, Senior Resident, Department of Orthopaedics, AIIMS, Jodhpur - 342 005, Rajasthan, India. E-mail: drkunal2408@gmail.com
Introduction: Numerous reflexive responses have been documented as alterations to the Babinski sign within upper motor neuron lesions. However, scant attention has been given to reflexes beyond these, which exhibit independence from the extensor plantar response. These reflexes predominantly form polysynaptic arcs, with nociceptive stimuli acting as afferents.
Case Report: The reflex was serendipitously discovered in an 18-year-old female patient who presented with spastic paraplegia with bowel and bladder involvement, as a consequence of an aneurysmal bone cyst of the D3 (dorsal) vertebrae, and the same was named after the authors as “Yadav–Kunal reflex” which can be defined as: “In individuals with spastic paraparesis, forcibly plantarflexing the toes will result in sudden jerky flexion of the knee and hip on the same side.” This novel reflex was further investigated and validated in two additional patients with spastic paraplegia: one, a 45-year-old female with D9-D10 Pott’s spine and bowel and bladder involvement, and the other, a 65-year-old male with D10-D11 compressive myelopathy and bowel and bladder involvement. This reflex was meticulously tracked until the abatement of spasticity following surgical intervention. Notably, its manifestation was evident in individuals experiencing spastic paraparesis, dissipating concomitantly with the resolution of spasticity – a direct clinical correlation. Conversely, the reflex was conspicuously absent in cases of flaccid paraplegia.
Conclusion: Spasticity, characterized by an increase in muscle tone on swift stretching movements, is a manifestation of a stretch reflex disorder. This condition is primarily induced by lesions affecting upper motor neurons. The activation of muscle spindles in toe dorsiflexors (primarily governed by the L5 nerve) occurs during forceful elongation caused by plantarflexion.
Keywords: Reflex arc, muscle spasticity, spinal cord compression, motor neuron disease, neurologic examination.
Numerous reflex modifications have been documented in association with the Babinski sign in cases of upper motor neuron (UMN) lesions. However, there has been limited exploration of reflexes beyond these, especially those that are completely independent of the extensor plantar response [1]. These reflexes typically involve a polysynaptic pathway and are elicited by a nociceptive stimulus as the afferent input [2,3]. In this study, we present a novel reflex (Videos 1,2,3) that diverges significantly from the previously described ones. Unlike the reflexes linked to Babinski’s sign, our observed reflex is entirely independent of the extensor plantar response, thus not qualifying as a mere modification of Babinski’s sign. In addition, its afferent input is derived from forced motor activity in a spastic muscle, rather than sensory stimulation.
Case 1
An 18-year-old female patient presented to the out-patient department (OPD) with complaints of bilateral lower limb weakness for 3 months and inability to walk for the past 2 months. On examination, she had complete spastic paraplegia with exaggerated deep tendon reflexes (3+) of the lower limb, no sensation below the T4 level, and no bowel-bladder control. Her plantar response was extensor. During clinical examination, a reflex was discovered serendipitously (Video 1).
On forced plantar flexion of all toes, a jerky flexion of the ipsilateral knee and hip (knee > hip) was observed. The reflex was repeatable. The intensity decreased when only the great toe was plantarflexed as compared to forced plantarflexion of all toes combined. The reflex was not demonstrable with ankle dorsiflexion or plantarflexion. She was further evaluated with MRI and suspected as a case of aneurysmal bone cyst (ABC) at D3 with paraparesis with bowel bladder involvement which was confirmed postoperatively on histopathology. The patient was planned for and underwent procedure in the form of posterior decompression (D2-D4) and excision of posterior elements of ABC. Post-surgery, her spasticity started decreasing and the intensity of reflex started decreasing from post-operative day 2 (<75° knee flexion). On post-operative day 1 the reflex still consisted of up to 90° knee flexion but not beyond. By 5th day, the reflex was nearly non-recordable. She was discharged on the 5th post-operative day and recalled for evaluation at the time of suture removal by which time the reflex was completely absent. By this time, her spasticity had decreased considerably and she had shifted to flaccid paraplegia with no signs of improvement in the sensory component. Her plantars were still extensor.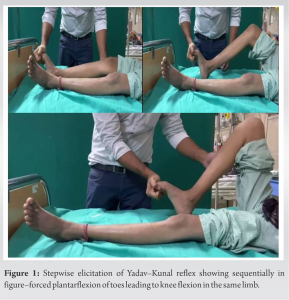
Case 2
A 45-year-old female patient presented to OPD with complaints of gradually increasing bilateral lower limb weakness for past 6 months and inability to walk for the past 10 days. On examination, she had complete spastic paraplegia with exaggerated deep tendon reflexes (3+) of the lower limb, decreased sensation below the D10 level, and no bowel-bladder control (decreased perianal sensation). Her plantar response was extensor. The above-mentioned reflex was tested on her due to the similarity in profile of paraplegia. The reflex was found to be repeatable and characteristics of reflex were found to be similar (Video 2).
She was further evaluated with MRI and suspected as a case of D9-D10 Pott’s spine and bowel and bladder involvement which was confirmed postoperatively on histopathology. The patient was planned for and underwent procedure in the form of posterior decompression (D9-10) and posterior instrumentation (D7-12) alongwith drainage of an abscess. Post- surgery, her spasticity started decreasing and the intensity of reflex started decreasing from post-operative day 1 itself (<75° knee flexion). By the 5th day, at the time of her discharge, the reflex was still appreciable. She was called for suture removal on day 14th by which time the reflex was completely absent (Video 4). By this time, her spasticity had completely vanished and the tone of the lower limbs was flaccid with slight improvement (subjective) in the sensory component but the sensory level was still the same. Her plantar reflex was equivocal bilaterally at this time.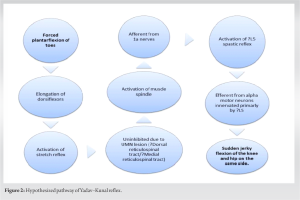
Case 3
A 65-year-old male patient presented to OPD with complaints of low back ache with bilateral claudication for 1 year which has increased to gradually increasing bilateral lower limb weakness for past 3 months. He was bedridden for the past 3 weeks following tightness in his lower limbs. On examination, he had complete spastic paraplegia with exaggerated deep tendon reflexes (4+) of the lower limb and intact sensation but no bowel-bladder control (normal perianal sensation). His plantar response was extensor. The reflex was repeatable (Video 3).
During the repetition of reflex, a unique aspect was observed that this produced clonus in the patient during demonstration. However, clonus was not observed in every demonstration and was usually present only when there was a slight gap between two demonstrations. This time gap was not quantified. There was no clonus with plantarflexion of the great toe. He was further evaluated with MRI and suspected as D10-D11 degenerative compressive myelopathy with severe ligamentum flavum hypertrophy and facet arthropathy. The patient was planned for and underwent procedure in the form of posterior decompression (D10-11) and posterior instrumentation (D9-D12). Post-surgery, his spasticity started decreasing and the intensity of reflex started decreasing from post-operative day 2 (<75° knee flexion). On post-operative day 1, the reflex still consisted of up to 90° knee flexion but not beyond. By 5th day, the reflex was still recordable. He was discharged on the 5th post-operative day and recalled for evaluation at the time of suture removal (day 15th) by which time the reflex was nearly absent which completely vanished at 1 month follow-up. By this time, his spasticity has also vanished and he has started gaining flickering of movement in the lower limb in L3-S1 distribution. The bowel and bladder control had not resolved fully, however, he was responding well to bowel and bladder training and reported occasional control. His plantar reflexes were still mute by this time.Yadav–Kunal Reflex (Fig. 1)
The reflex can be described as follows: “In individuals with spastic paraparesis, forcibly plantarflexing the toes will result in sudden jerky flexion of the knee and hip on the same side.”
Presentation and validation of reflex
The reflex was serendipitously discovered in an 18-year-old female patient who presented with spastic paraplegia with bowel and bladder involvement, as a consequence of ABC of the D3 (dorsal) vertebrae (Fig. 1). This novel reflex was further investigated and validated in two additional patients with spastic paraplegia: one, a 45-year-old female with D9-D10 Pott’s spine and bowel and bladder involvement, and another, a 65-year-old male with D10-D11 compressive myelopathy and bowel and bladder involvement. In contrast, the reflex was not observed in two other patients with traumatic paraplegia, who had flaccid paralysis (not presented here as reflex was not demonstrable–these were only used for validation purposes). All patients with this reflex had at least 3+ deep tendon reflexes and extensor plantar response, in addition to spastic paraplegia. No clonus with toe plantar flexion validated further the hypothesis that toes plantar flexion produced a reduced intensity of reflex as compared to plantar flexion of all toes together. During the reflex, the knee was fully flexed, while the hip was not flexed beyond 90°. In one patient, the jerky movement included an ill-sustained clonus (three continuous jerks), but this clonus was not consistently reproduced in further testing or observed in other patients. In addition, this particular patient showed slight hip abduction, which might be explained by the possibility of a higher grade of reflex, involving clonus and affecting other muscle groups supplied by the L5 nerve root, such as the hip abductors. This higher grade could be due to the comparably longstanding nature (>1 year) of compression in this patient as compared to the other two in whom the symptoms have been <6 months. Moreover, the reflex was not elicited by forced ankle dorsiflexion/plantarflexion, and only partial responses were observed with forced plantarflexion of the great toe. After successful decompression surgery, the reflex resolved concomitantly with the spasticity. Remarkably, it was the first reflex to respond to the loss of spasticity, decreasing dramatically in proportion to the reduction in spasticity. This reduction in reflex activity was observed before the resolution of clonus and extensor plantar responses, becoming evident as early as day 1 post-surgery (measured by knee flexion, which was limited to 75–90° in all cases) and almost completely resolving by day 15 (with <10° of knee flexion).
The characteristics of the reflex can be summarized as:
- The reflexive response corresponds directly to the degree of spasticity, dwindling proportionally with its amelioration, culminating in complete resolution with a resolution of spasticity following the intervention.
- The reflex remains absent in instances of flaccid paraplegia.
- A distinctive feature of the reflex entails a motor stimulus as its afferent source, wherein the toes’ plantarflexion serves as the trigger, whereas endeavors in ankle dorsiflexion/plantarflexion fail to evoke the response comprehensively. Furthermore, partial responses are elicited by exclusive plantarflexion of the great toe.
- In terms of efferent output, the reflex engenders limb withdrawal, wherein knee flexion predominantly supersedes hip flexion and hip abduction. Importantly, the hip flexion synchronously aids complete knee flexion, remaining consistently constrained within 90° in all cases.
- The reflex can be elicited with great toe plantarflexion but with reduced intensity. The reflex can also generate a clonus response if the deep tendon reflexes (DTR) are producing a clonus.
- In contrast to DTR, the response is not elicited in the same muscle but probably involves a reflex arc involving the spinal cord eliciting the response in a different group of muscles but a similar myotome.
In compliance with the guidelines provided by Escobar et al., and used by Benavides and Castro for the evaluation of a test by a panel of experts, the reflex testing was assessed in four different dimensions [4,5]. These aspects looked at the test’s application time, ease of demonstration, efficacy, and clinical value. The ratings were as follows: 1–3 for time (long, acceptable, or short application time); 1–2 for ease of demonstration (difficult or easy to execute); 1–3 for efficacy (1 for partially replicable, 2 for fully replicable without clonus, 3 for fully replicable including clonus); and 1–2 for clinical value (test was useful or was it not in relation to clinical spasticity). After adding together all of the numbers, a score between 4 (the lowest score) and 10 (the greatest score) was generated. The reflex testing was further classified as undesirable (score <6), acceptable (score 6–8), or exceptional (score >8) based on the establishment of a cutoff threshold. It was scored both before and after surgery, that is, when spasticity was present before surgery and after surgery. A score of at least 9 was found on both occasions (3 + 2 + 2 + 2).
Hypothesized pathophysiology
Spasticity being an aberrant manifestation of the stretch reflex, exhibits an escalating muscular tone during rapid stretching, predominantly arising from upper motor neuron lesions [2,6]. In physiologically intact individuals, the stretch reflex constitutes the bedrock of deep tendon reflexes, giving rise to muscle contractions [7]. Notably, passive muscle stretching triggers activation of the muscle spindle, eliciting discharges from Ia fibers, which subsequently transmit inputs to alpha motor neurons through largely monosynaptic pathways. These alpha motor neurons, in turn, execute efferent impulses that orchestrate muscle contractions [8].
Hypothesized pathway (Fig. 2)
Forceful plantar flexion of toes leads to forceful stretching of the toe dorsiflexors which are predominantly governed by the L5 nerve root, culminates in their muscle spindle activation, vividly demonstrated through sudden toe plantarflexion in the reflex [9]. This activation subsequently triggers muscle contractions in L5-innervated muscles. Ordinarily, inhibitory signals originating from tracts above L5 would repress the spastic response in other L5-innervated muscles, ensuring normative posture and gait. However, the UMN lesion above L5 precipitates the attenuation of these inhibitory influences, fostering knee joint flexion, notably mediated by the hamstrings (predominantly innervated by L5-S2). The spasticity might emanate from impeded dorsal reticulospinal tract functionality, known to exert inhibitory control. In complete injuries engendering flaccid paraplegia, both inhibitory dorsal reticulospinal and facilitatory medial reticulospinal control might be compromised [6,10]. Zhu et al. have illuminated that stimulation spanning L5 to S2 predominantly incites the extensor digitorum brevis, whereas biceps femoris predominantly derives its innervation from L4 to S2, and gluteus maximus primarily from L4 and L5, with supplemental contributions from S1 [11]. The absence of reflexive response to ankle plantar/dorsiflexion, and the discernibly augmented response to plantarflexion encompassing all toes relative to the great toe singularly, suggests the primacy of the L5 nerve root’s involvement.
Application
The unearthing of this reflex harbors the potential to disclose novel reflexes capable of discerning UMN lesions from lower motor neuron lesions. Whereas Babinski’s sign may yield various responses, the Yadav–Kunal reflex consistently offers a uniform response across all cases of spastic paraparesis. Moreover, it stands as the foremost reflex to subside in tandem with spasticity, mirroring its clinical magnitude, objectively gauged by muscle tone. Although not subjected to grading in this study, the subjective assessment of knee flexion could conceivably serve as a metric to quantify spasticity, while retaining the objectivity of muscle tone assessment. This reflex further underscores that nociceptive stimuli need not invariably be sensory in nature; rather, a motor stimulus might engender pain within spastic muscles, thereby accentuating the muscle stretch reflex [12]. Furthermore, since both afferent and efferent are motor, theoretically (unlike Babinski’s reflex) they should not be affected by the presence/absence of sensory status of an individual.
Further Research
Promising follow-up investigations are warranted to authenticate the proposed mechanism, validate findings across a broader patient cohort, and explore potential variations in response, such as the presence of clonus or the involvement of other muscle groups primarily innervated by L5, such as the hip abductors.
The conspicuous manifestation of spasticity, concomitant with the relinquishment of spinal inhibition for exciting other muscle groups, commonly innervated by an L5 root within UMN lesions, engenders the Yadav–Kunal reflex. This reflex emerges as not only a plausible determinant of spasticity severity but also as a gateway to unraveling novel reflexes governed by motor afferents in future investigations.
The demonstrated reflex can be used to quantify spasticity and recovery from the lesion clinically following intervention.
References
- 1.Ambesh P, Paliwal VK, Shetty V, Kamholz S. The Babinski sign: A comprehensive review. J Neurol Sci 2017;372:477-81. [Google Scholar | PubMed]
- 2.Sheean G. The pathophysiology of spasticity. Eur J Neurol 2002;9:3-9, dicussion 53-61. [Google Scholar | PubMed]
- 3.Bassetti C. Babinski and babinskis sign. Spine (Phila Pa 1976) 1995;20:2591-4. [Google Scholar | PubMed]
- 4.Escobar-Pérez J, Cuervo-Martínez Á. Validez de contenido y juicio de expertos: Una aproximación a su utilización. Adv Med 2008;6:27-36. [Google Scholar | PubMed]
- 5.Benavides J, Castro OE. Validation of a semiological maneuver for the evaluation of spastic dynamic clubfoot: An approach based on the judgment of experts in physical medicine and rehabilitation. Eur J Phys Rehabil Med 2022;58:575-83. [Google Scholar | PubMed]
- 6.Trompetto C, Marinelli L, Mori L, Pelosin E, Currà A, Molfetta L, et al. Pathophysiology of spasticity: implications for neurorehabilitation. Biomed Res Int 2014;2014:354906. [Google Scholar | PubMed]
- 7.Hall JE, Hall ME. Guyton and Hall Textbook of Medical Physiology E-Book. Netherlands: Elsevier Health Sciences; 2020. [Google Scholar | PubMed]
- 8.Fitz-Ritson D. The anatomy and physiology of the muscle spindle, and its role in posture and movement: A review. J Can Chiropr Assoc 1982;26:144-50. [Google Scholar | PubMed]
- 9.Roberts TT, Leonard GR, Cepela DJ. Classifications in brief: American spinal injury association (ASIA) impairment scale. Clin Orthop Relat Res 2017;475:1499-504. [Google Scholar | PubMed]
- 10.Engberg I, Lundberg A, Ryall R. Reticulospinal inhibition of transmission in reflex pathways. J Physiol 1968;194:201-23. [Google Scholar | PubMed]
- 11.Zhu L, Lin HD, Chen AM. Accurate segmental motor innervation of human lower-extremity skeletal muscles. Acta Neurochirur (Wien) 2015;157:123-8. [Google Scholar | PubMed]
- 12.Matre DA, Sinkjær T, Svensson P, Arendt-Nielsen L. Experimental muscle pain increases the human stretch reflex. Pain 1998;75:331-9. [Google Scholar | PubMed]








