[box type=”bio”] Learning Point of the Article: [/box]
Spinal schwannoma excision has a good surgical outcome despite the late presentation with neurological involvement.
Case Report | Volume 10 | Issue 2 | JOCR March – April 2020 | Page 101-105 | Ed Simor Khan Bin MorJapar Khan, Charles Ang Poh Thean, Zamzuri Bin Zakaria, Mohamed Saufi Bin Awang, Rajandra Kumar Karupiah, MohdShukrimi Bin Awang. DOI: 10.13107/jocr.2020.v10.i02.1718
Authors: Ed Simor Khan Bin MorJapar Khan[1], Charles Ang Poh Thean[1], Zamzuri Bin Zakaria[1], Mohamed Saufi Bin Awang[2], Rajandra Kumar Karupiah[1], MohdShukrimi Bin Awang[1]
[1]Department of Orthopaedics, International Islamic University Malaysia Medical Centre (IIUM MC), Jalan Sultan Ahmad Shah, 25200 Kuantan, Pahang, Malaysia,
[2]Department of Neurosurgery, International Islamic University Malaysia Medical Centre (IIUM MC), Jalan Sultan Ahmad Shah, 25200 Kuantan, Pahang, Malaysia.
Address of Correspondence:
Dr. Charles Ang Poh Thean,
Department of Orthopaedics, International Islamic University Malaysia Medical Centre (IIUM MC), Jalan Sultan Ahmad Shah, 25200 Kuantan, Pahang, Malaysia.
E-mail: capt85@hotmail.my
Abstract
Introduction: Spinal schwannoma can occur anywhere along the spinal cord but is predominantly seen in the cervical and thoracic region.It composes mainly of well-differentiated schwann cell and is benign in nature. It is typically seen in the peripheral nerves and is commonly associated with neurofibromatosis. Up to 80% of cases, spinal schwannoma is reported to be intradural in location and 15% of cases have both intradural and extradural components. Spinal schwannoma rarely causes conus medullaris syndrome.
Case Report: In this case series, all three female patients in their 4th and 5th decades of life presented with conus medullaris syndrome. Lower back pain, radiculopathy, lower limb weakness, and urinary incontinence are their main clinical presentation. Magnetic resonance imaging shows a well-defined intradural, extramedullary mass compressing onto the conus medullary region. These patients undergone microscopic assisted excision of the tumor and had remarkably good early outcome despite the advanced presentation of neurological deficit.
Conclusion: Despite the late presentation with significant neurological deficit, surgical excision of spinal schwannomas carries a good prognosis postoperatively due to their benign nature and extramedullary location.
Keywords: Benign extramedullary lesion, conus medullaris syndrome, spinal schwannoma.
Introduction
Spinal tumors account for 5–10% of all central nervous system tumors. Of this, 70–80% are intradural extramedullary in location. Schwannomas and meningiomas are the most common tumors to occur in this location [1]. Majority benign schwannomas are completely capsular with well-defined margins and clear boundaries [2]. Schwannomas are benign and slow-growing nerve sheath tumors, originating from the neural crest. It composes mainly of well-differentiated Schwann cells typically seen in the peripheral nerves and is commonly associated with neurofibromatosis. They are usually located intradural, extramedullary, or extradurally. It typically manifests in the 4th–5th decades of life with predilection for men [3]. However, some literature cites no gender predilection. During the early phase, it rarely has any clinical signs and symptoms. Diagnosis is usually late when the patient presents with neurological signs and symptoms. The main presentations are pain, motor and sensory changes with sphincter disturbances. In this case series, all three patients presented late with these symptoms, incontinence being the tipping point of presentation.
Case Report
Case 1
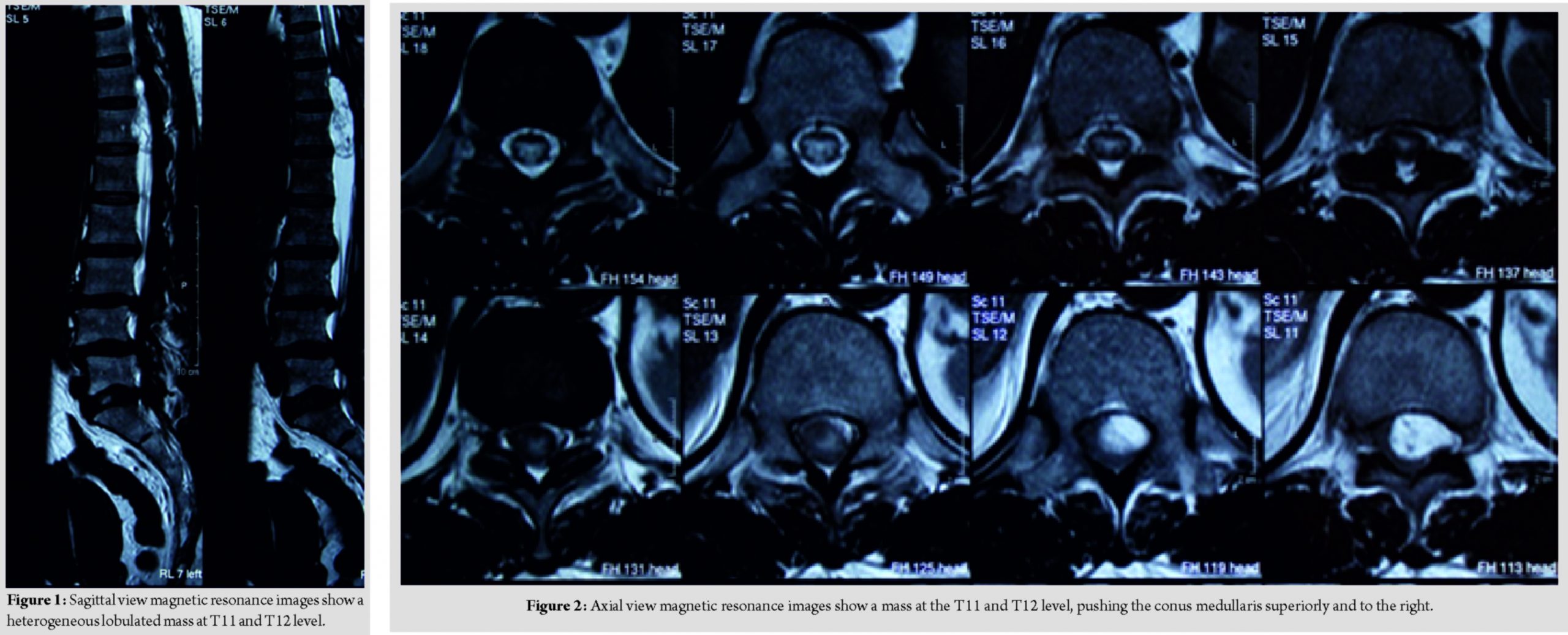
A 57-year-old female lady presented with complaint of sudden onset of urinary incontinence for 3 days duration. Further, history taking reveals that she has been having radiculopathy pain and bilateral lower limb weakness for a year duration. Clinical examination reveals that bilateral lower limb hypotonia, bilateral lower limb motor power of Medical Research Council (MRC) Grade 2, and bilateral lower limb reflexes were absent and sensory reduced from T11 dermatome onward. Magnetic resonance imaging reveals a heterogeneous lobulated mass in the spinal canal at T11 and T12 level, compressing and displacing the conus medullary superiorly and to the right (Fig. 1 and 2).
She underwent laminectomy of T11 and T12 with microscopic assisted excision of the tumor. The mass was located intradural extramedullary and was successfully removed completely; however, the nerve root had to be sacrificed (Fig. 3 and 4).
At post-operative day 1, the radiculopathy pain resolved. She regained bladder control at the 1st week post-operative. She was ambulating with aid at 2 weeks post-operative and by 2 months, she was able to walk unaided. There was no detectable post-operative neurological deficit despite sacrificing the involved nerve root. The final diagnosis was confirmed through histopathological examination.
Case 2
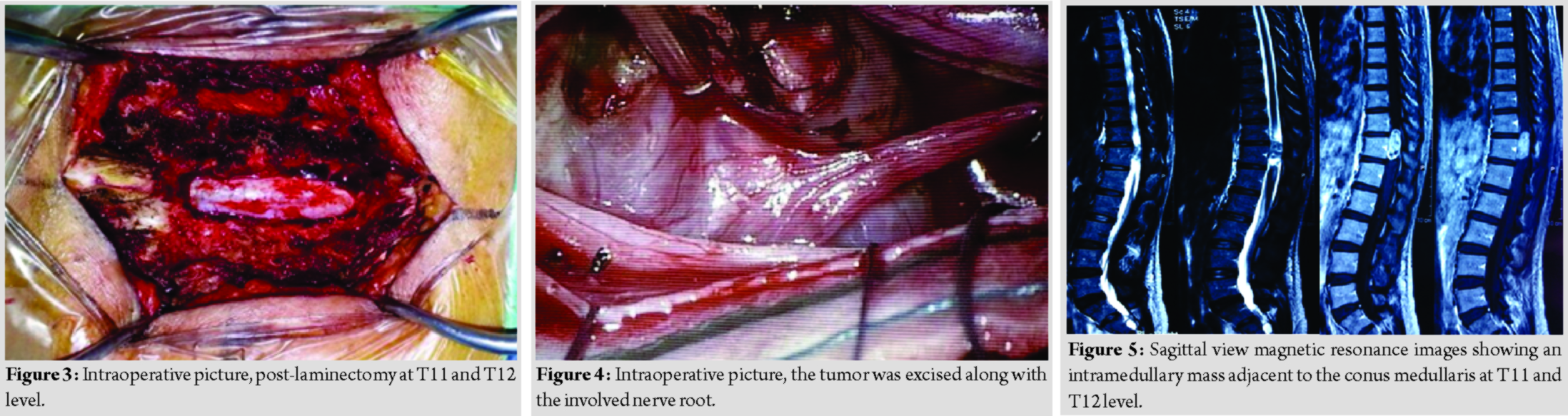
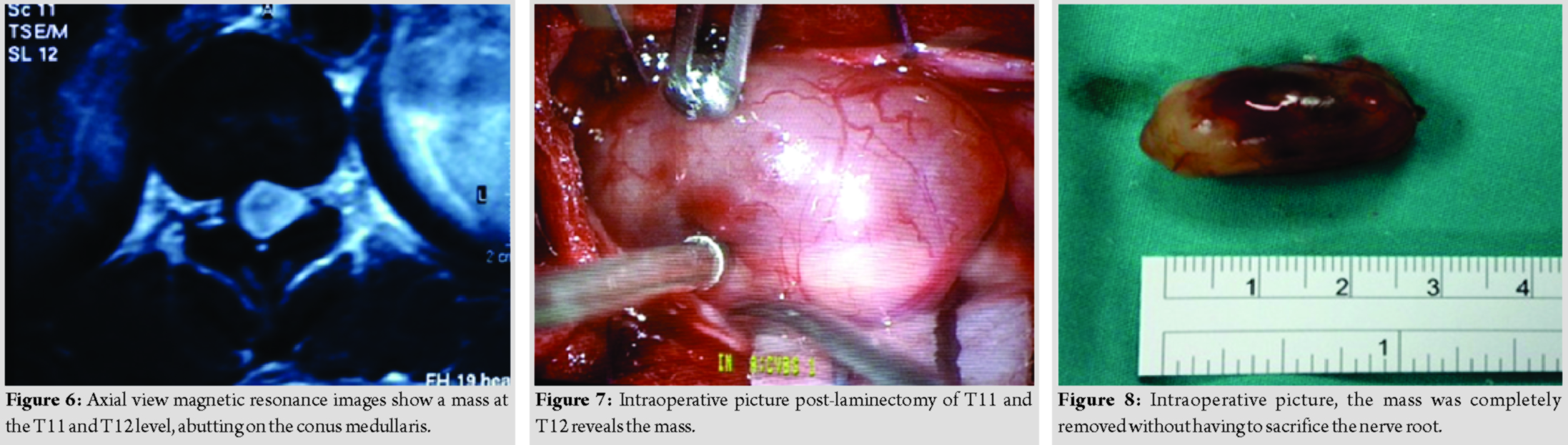
A 44-year-old lady presented with back pain and bilateral lower limb weakness for 2 years duration. She then progressed to develop urinary incontinence for 2 months duration. Clinical examination reveals bilateral lower limb hypertonia, lower limb power was Grade 0, and reflexes were brisk, sensory reduced from T11 dermatome onward. Magnetic resonance imaging reveals an intramedullary mass adjacent to the conus medullary (Fig. 5 and 6). Differential diagnosis at that point of time was astrocytoma and ependymoma. She underwent laminectomy of T11 and T12 with microscopic assisted tumor excision (Fig. 7 and 8). The mass was removed completely without having to sacrifice the involved nerve root. At 1-week post-operative, she was able to ambulate with aid. At 1-month post-operative, she was able to ambulate without aid, regained her bladder control and back pain resolved. Final histopathological findings reveal schwannoma.
Case 3
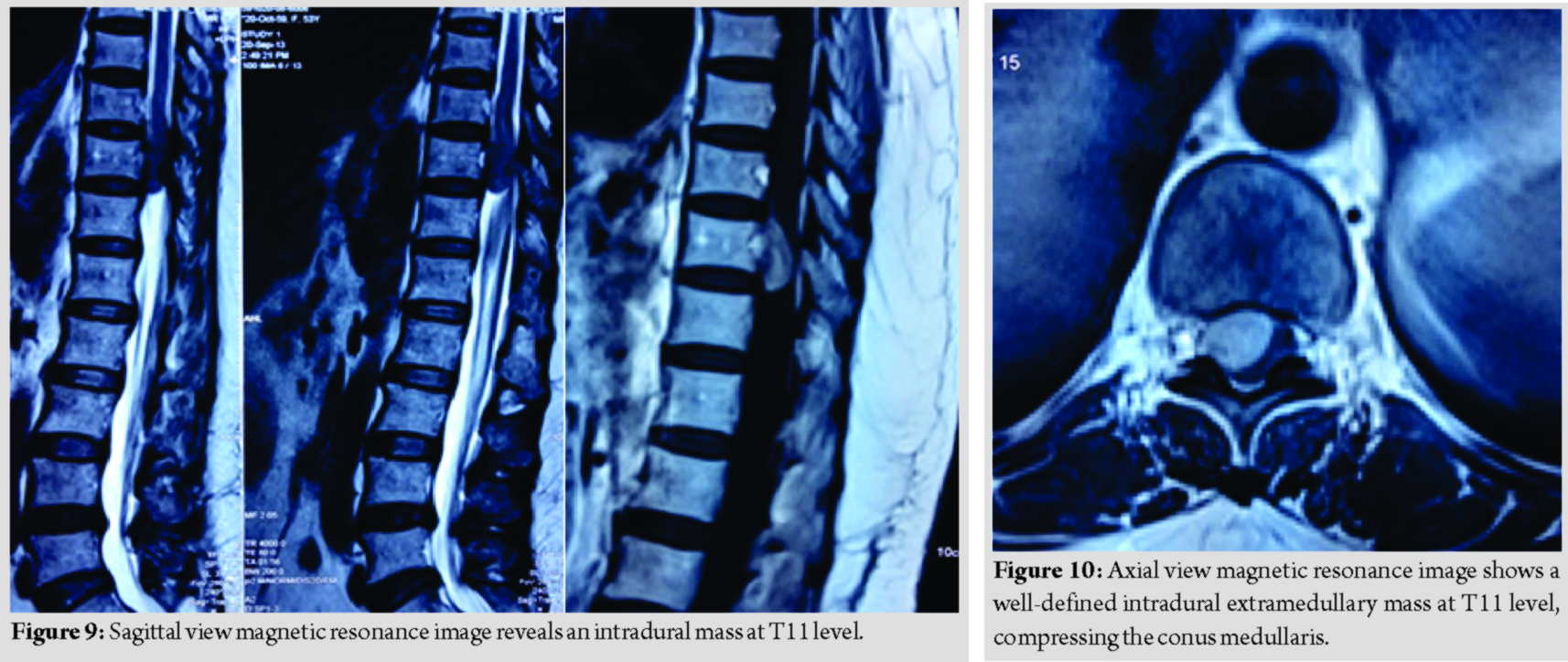
A 53-year-old lady presented with complaint of back pain and bilateral lower limb weakness for a year duration, associated with urinary incontinence for 2 months duration. Examination reveals normal tone, power over the right lower limb was more affected (MRC Grade 2) as compared to the left lower limb (MRC Grade 3), lower limb reflexes were brisk, and sensation was reduced from T11 dermatome onward. Magnetic resonance imaging reveals a well-defined intradural extramedullary mass at T11, compressing on the conus medullaris (Fig. 9 and 10).
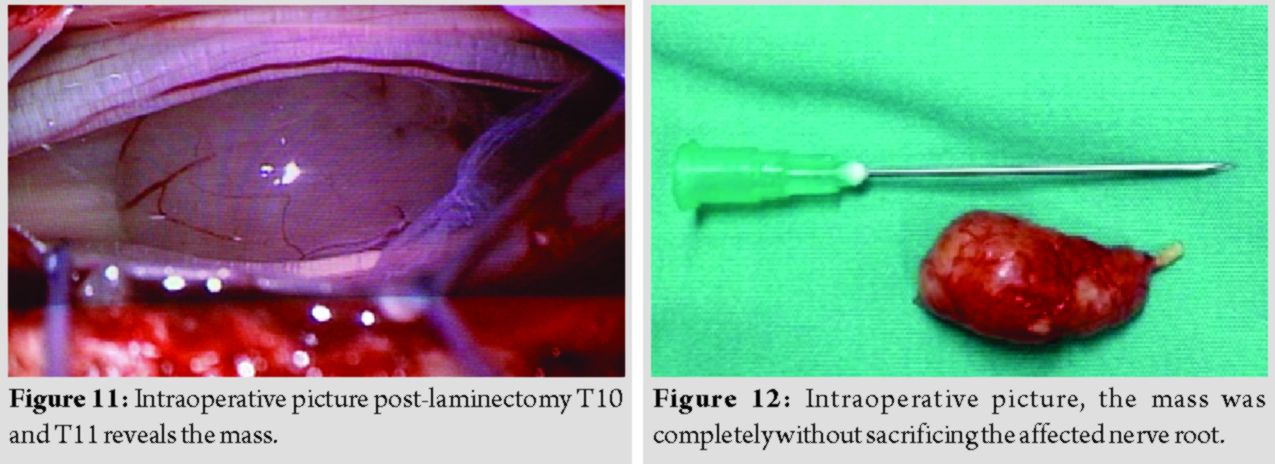
She underwent laminectomy of T10 and T11 with microscopic assisted excision of the tumor. The mass was successfully removed completely without sacrificing the involved nerve root (Fig. 11 and 12).
Her neurological recovery was remarkable despite being symptomatic for a year duration. By post-operative day 2, she claims that her back pain has completely resolved. She regained bladder control at 1-week post-operative and was ambulating with aid. At 2 weeks post-operative, she was able to ambulate without aid. Final diagnosis was confirmed through histopathology. None of these patient suffered from neurofibromatosis. No instrumentation was done to stabilize the spine as only a single-level laminectomy was involved. The surgeon took careful measures to ensure the facet remains untouched and thereby maintaining the stability of the spine. These patients have followed up for the past 7 years with no progressive kyphosis noted or clinical symptoms and signs suggesting recurrence.
Discussion
Schwannomas are typically slow-growing, benign, nerve sheath tumor of neural crest origin, compose almost entirely of well-differentiated Schwann cells and usually occur in the peripheral nerves. This explains its common intradural extramedullary location. In this case series, the average time of presentation from the onset of initial symptoms was 18 months. Literature review shows a duration of 1–72 months from onset of clinical symptoms until admission and treatment. Morbidity and mortality rate was reported to be from 0 to 6%. Almost all patient reports marked neurological improvement following surgical excision of the tumor. Albanese and Platania analyzed 41 surgically treated patients with intradural extramedullary spinal tumor. He found that up to 60% achieved significant neurological improvement and recommended radical surgery as the ideal goal in schwannoma excision for the best long-term results [4]. Imaging studies are important to assist in diagnosis and treatment planning. Magnetic resonance imaging of intradural schwannomas shows signal intensity equal to or less than that of the spinal cord on T1-weighted images and hyperintense on T2-weighted images. Focal area of greater hyper-intensity corresponds to cystic portions and hypointensity represents hemorrhage, dense cellularity, or collagen deposition. These heterogeneous appearances do not necessarily indicate malignant changes. Schwannomas are usually posteriorly located tumor with well-defined plane of cleavage. This makes complete resection of the tumor possible, thereby providing the best clinical outcome and reduces the chances of recurrence. The rate of recurrence is reported to be <5% in patients that have undergone surgical excision and usually occurs several years after the initial surgery [5]. To date, none of these patients in the case series develop clinical symptoms or signs suggesting recurrence. Surgical excision of the schwannoma with preservation of the involved nerve root is generally recommended. However, this may not be possible to achieve in certain cases, especially in large tumors. In cases where complete excision is not feasible, sacrificing the involved nerve root is advocated to achieve complete excision. Resection of the involved nerve root does not always result in post-operative neurological deficit[6]. A retrospective study by Kim et al. on 86 cases of spinal schwannoma that undergone surgical excision, 31 of these cases ultimately required sacrificing of the involved nerve root. However, only seven out of 31 cases (23%) developed motor or sensory deficit postoperatively. These deficits were no more than partial loss of strength or sensation. With this, the author suggest that majority of these involved nerve root are usually non-functional at the time of surgery and sacrificing the nerve carries only a small risk of post- operative neurological deficit. Jeon et al. cited two main obstacles faced to achieve complete resection, namely, adhesion of the tumor to the spinal cord due to hemorrhage, inflammation, or subpial localization. The other is due to critical structures attached to the extradural components outside the spinal cord such as the vertebral artery. As for the decision of laminoplasty versus laminectomy during tumor excision, the degree of kyphotic deformity following laminectomy correlates with the removal of facet joints. If bilateral facets are removed at one or more levels, acute angular kyphosis tends to occur. Unilateral facetectomy may also predispose to angular kyphoscoliosis ultimately lead to spinal cord compression despite the lack of tumor recurrence [7]. However, the development of gradual kyphosis is also seen despite no facetectomy done. A study by Kim et al. supported laminoplasty over laminectomy done during the time of tumor excision, in preventing post-operative spinal deformities citing only 16 cases of laminoplasty as compared to 81 cases of laminectomy developed spinal deformities postoperatively [8]. Kawahara et al. reported no increase in complication rate such as post-operative spinal canal stenosis, facet arthrosis, or kyphosis following recapping of T-saw laminoplasty done for spinal cord tumor removal [9]. Jeon et al. recommended that unilateral facetectomy done in adults for tumor excision at the cervical or thoracic spine does not require fusion, but may be necessary for the lumbar spine. Complete removal of schwannomas that are not associated with neurofibromatosis is generally curative [10]. As seen in this case series, where none of these patient suffered from neurofibromatosis and after 7 years of follow-up, none showed any clinical symptoms and signs suggesting recurrence. Microsurgery is the main modality of spinal tumor treatment with the aim of obtaining a complete removal of the tumor [11]. The histology findings of these neoplasms explain it’s heterogeneous appearance. Schwannomas compose of both Antoni type A and type B patterns, where Antoni type A is packed with cells and compose of compact bundles of fibrillated cells and Antoni type B tissue lacks of cells and is composed mainly of loose stromal tissue, in addition, cystic degeneration and xanthomatous changes may be present [12].
Conclusion
Spinal schwannoma can occur anywhere along the spinal cord but is predominantly seen in the cervical and thoracic region. Rarely, it can become significant in size compressing the spinal cord causing neurological deficit. In the thoracic region it carries a risk of causing conus medullaris syndrome as seen in this case series. Due to its benign nature, the presentation is usually late with advanced neurological deficit.
Clinical Message
Microscopic assisted surgical excision of intradural extramedullary spinal schwannoma carries a good prognosis despite the late presentation with significant neurological deficit. This is due to the benign nature of the neoplasm and its extramedullary location. The well-defined plane of cleavage allows the tumor to be completely excised. In addition, majority of the involved nerve roots are sensory in origin and are usually non-functional at the time of excision and sacrificing it at the time of surgery carries a small risk of post-operative neurological deficit.
References
1. KoellerKK, ShihRY. Intradural extramedullary spinal neoplasms: Radiologic-pathologic correlation.Radiographics2019;39:468-90.
2. ZhangE, ZhangJ, LangN, YuanH. Spinal cellular schwannoma: An analysis of imaging manifestation and clinicopathological findings.Eur J Radiol2018;105:81-6.
3. SinghR, ChaturvediS, PantI, SinghG, KumariR. Intramedullary schwannoma of conus medullaris: Rare site for a common tumor with review of literature.Spinal Cord Ser Cases2018;4:99.
4. AlbaneseV, PlataniaN. Spinal intradural extramedullary tumors. Personal experience.J Neurosurg Sci2002;46:18-24.
5. DhakeRP, ChatterjeeS. Recurrent thoracic intramedullary schwannoma: Report of two cases with long term follow up.Br J Neurosurg2019;10:1-4.
6. KimP, EbersoldMJ, OnofrioBM, QuastLM. Surgery of spinal nerve schwannoma. Risk of neurological deficit after resection of involved root.J Neurosurg1989;71:810-4.
7. LonsteinJE. Post-laminectomy kyphosis.Clin OrthopRelat Res1977;128:93-100.
8. Kim SH, Chin DK, Yoon YS, Jin BH, Cho YE, Kim YS. Spinal instability following for spinal cord tumors: Laminoplasty versus laminectomy. J Korean Neurosurg Soc 2001;30:61-7.
9. KawaharaN, TomitaK, ShinyaY, MatsumotoT, BabaH, FujitaT, et al. Recapping T-saw laminoplasty for spinal cord tumors.Spine (Phila Pa 1976)1999;24:1363-70.
10. Jeon JH, Hwang HS, Jeong JH, Park SH, Moon JG, Kim CH. Spinal schwannoma; Analysis of 40 cases. J Korean Neurosurg Soc 2008;43:135-8.
11. Guerrero-SuarezPD, Magdaleno-EstrellaE, Guerrero-LópezP, Vargas-FigueroaAI, Martínez-AndaJJ. Intradural spinal tumors: 10 years surgical experience in a single institution.Clin Neurol Neurosurg2018;169:98-102.
12. FriedmanDP, TartaglinoLM, FlandersAE. Intradural schwannomas of the spine: MR findings with emphasis on contrast-enhancement characteristics.AJR Am J Roentgenol1992;158:1347-50.
 |
 |
 |
 |
 |
 |
| Dr. Ed Simor Khan Bin Mor Japar Khan | Dr. Charles Ang Poh Thean | Dr. Zamzuri Bin Zakaria | Dr. Mohamed Saufi Bin Awang | Dr. Rajandra Kumar Karupiah | Dr. Mohd Shukrimi Bin Awang |
| How to Cite This Article: Ed Simor Khan MJK, Charles APT, Zamzuri BZ, Saufi MBA, Rajandra KK, Shukrimi BA. A Rare Presentation of Spinal Schwannoma Causing Conus Medullaris Syndrome: A Case Series on Surgical Outcome. Journal of Orthopaedic Case Reports 2020 Mar-Apr;10(2): 101-105. |
[Full Text HTML] [Full Text PDF] [XML]
[rate_this_page]
Dear Reader, We are very excited about New Features in JOCR. Please do let us know what you think by Clicking on the Sliding “Feedback Form” button on the <<< left of the page or sending a mail to us at editor.jocr@gmail.com