In Fibrous dysplasia involving more than half the length of radius bone, a tightly fitting non-vascularised fibular cortical strut graft jammed into the defect caused by lesion excision will suffice and there will be no need of any implant for fixation.
Dr. Nikhil Tandon, Department of Orthopaedics, Seth VC Gandhi and MA Vora Municipal General Hospital (Rajawadi Hospital), Mumbai - 400 077, Maharashtra, India. E-mail: nikhil.tandon.nt@gmail.com
Introduction: Fibrous dysplasia (FD) is a congenital disorder in which the bone is distorted and replaced by poorly organized and structurally unsound fibrous tissue. The disorder can be localized to a single bone or affects multiple bones. Although any bone can be affected, the bones of the upper extremity are less commonly involved by the disease. The disease process results in deformity of the bones and is often complicated by pathological fractures.
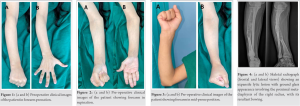
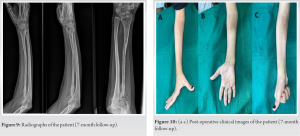
Case Report: A 14-year-old girl presented with gradually progressive deformity of the right forearm for the past 1 year associated with mild pain. Skeletal radiographs of the right forearm revealed an expansile lytic lesion with ground glass appearance involving the proximal meta-diaphysis of the right radius, with its resultant bowing. The zone of transition was narrow and there was no evidence of matrix calcification. The lesion was causing thinning of the bony cortex. With this radiographic appearance in mind, a diagnosis of FD of the radius was put forth. The lesion was managed surgically. The proximal three-fourth of the radius bone was exposed and the lesion was excised along with 1 cm of normal bone on the distal side. Proximally, a thin shell of the cortex was preserved after curettage of the proximal end of the radius. Fibular cortical strut graft was harvested from the leg of same side. Graft length was kept 2 cm more than the excised bone to avoid shortening of the forearm. The graft was beveled on the distal end and jammed into the shaft of the distal radius such that 1 cm of graft was inside the original bone. A long arm or above elbow splint was applied keeping the elbow at 90 degrees of flexion and the forearm in supination for a total of 6 months. The patient was being followed up regularly. Follow-up radiographs obtained at 7 months revealed complete incorporation of the cortical bone graft with reformation of the intramedullary bone canal and restoration of hand and elbow function.
Conclusion: Non-vascularized fibular cortical strut grafting is an effective treatment modality for FD of radius bone. External or internal fixation is not necessary if a tightly fitting cortical graft is jammed into the defect caused by lesion excision.
Keywords: Fibrous dysplasia, radius, cortical bone graft.
Fibrous dysplasia (FD) was a term first used by Lichtenstein in 1938 to designate a developmental anomaly of unknown etiology characterized by fibrous tissue replacement of the medullary cavity of bones [1]. It is a sporadic disease and may account for about 2.5% of bone disorders and 7% of so-called benign bone tumors [2]. The disorder is caused by activating mutations of alpha-subunit of Gs protein (GNAS) [3]. Most patients with FD are asymptomatic and are often diagnosed when a radiograph is performed for another reason. Any bone can be involved, depending on the form of the disease. FD can be limited to a single bone in its monostotic form or may be polyostotic involving several bones. Polyostotic FD is often monomelic; one side of the body is generally preferentially involved [4]. Polyostotic FD is associated with McCune-Albright syndrome and other endocrinal disturbances.
Although any bone can be involved, it is commonly found in the femur, tibia, ribs, and the bones of the jaw and skull. In the upper extremity, the humerus is most commonly involved. To date, only a few isolated case reports of FD involving the radius bone have been reported [5]. The disorder can be asymptomatic or may present with pain, swelling, stiffness, and deformity. Pain is often the consequence of an incomplete fracture, being either spontaneous or triggered by a minor trauma. The intensity of the pain is variable, ranging from minimal to excruciating, and can be activated by palpation [6]. The shape of all long bones can be altered, giving a shepherd’s crook deformity to the femur or femur [6]. Smaller lesions, particularly of the monostotic variety, can be left untreated. Treatment approach for symptomatic lesions or polyostotic FD includes curettage with bone grafting. With this report, we aim to present a rare case of FD of the radius bone treated with excision and non-vascularized fibular cortical bone graft without fixation.
A 14-year-old girl presented with gradually progressive deformity of the right forearm since the past 1 year associated with mild pain (Fig. 1, 2, 3). There was no history of localized trauma or fever. Family history for similar deformity or swelling was absent. Local examination revealed a palpable thickening with deformity of the proximal two-thirds of the right radius. There was restriction of terminal forearm pronation. However, function of the elbow and hand was unaffected. There was no evidence of distal neurovascular or tendon deficit.
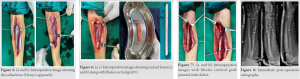
Skeletal radiographs (frontal and lateral view) of the right forearm revealed an expansile lytic lesion with ground glass appearance involving the proximal meta-diaphysis of the right radius, with its resultant bowing (Fig. 4a, 4b). The zone of transition was narrow and there was no evidence of matrix calcification. The lesion was causing thinning of the bony cortex. No obvious cortical breach, fracture, or periosteal reaction were noted. The overlying soft tissue was intact. Skeletal survey revealed no similar lesions elsewhere. With this radiographic appearance in mind, a diagnosis of FD of the radius was put forth. The patient’s symptoms and deformity warranted surgical intervention. In FD, cancellous bone grafts undergo resorption and replacement with the same type of poorly formed woven bone and thus recurrence occurs, which may lead to fracture and deformity. Hence, a different surgical approach was envisioned with the use of non-vascularized cortical bone graft. The lesion in the radius was explored using the Henry’s approach [7] (Fig. 5a, 5b). Inter-muscular interval being flexor carpi radialis and brachioradialis while inter-nervous plane being median nerve and radial nerve. The proximal three-fourth of the radius bone was exposed and the lesion was excised along with 1 cm of normal bone on the distal side (Fig. 6a, 6b). Proximally, a thin shell of the cortex was preserved after curettage of the proximal end of the radius. Fibular cortical strut graft was harvested from the leg of same side (Fig. 6c). Graft length was kept 2 cm more than the excised bone to avoid shortening of the forearm. The graft was beveled on the distal end and jammed into the shaft of the distal radius such that 1 cm of graft was inside the original bone (Fig. 7a, 7b). Immediate post-operative radiographs were obtained (Fig. 8) and a long arm or above elbow splint was applied keeping the elbow at 90 degrees of flexion and the forearm in supination for a total of 6 months.
The patient was being followed up regularly. Follow-up radiographs (Fig. 9) obtained at 7 months revealed complete incorporation of the cortical bone graft with reformation of the intramedullary bone canal and restoration of hand and elbow function (Fig. 10a-c).
FD is a congenital disorder in which osseous changes are characterized by the replacement and distortion of normal bone with poorly organized, structurally unsound, and fibrous tissue. The disease process may be localized to a single or multiple bones [8]. Cases of monoostotic FD are often asymptomatic, while the polyostotic form is usually diagnosed during the first few years of life. Radiographs play an important role in making an early diagnosis of these cases and are also helpful in their follow-up. Classically, lesions of FD are intramedullary, expansile, and show varying degrees of hazy density giving a ground glass appearance [4]. The lesions can vary in size ranging from a small, focal abnormality to a large lesion, perhaps involving most, or all of a long bone. There is cortical thinning with bowing and deformity of the involved bones. Lesions of FD can be complicated by pathological fracture, development of secondary aneurysmal bone cyst and rarely, malignant transformation to osteosarcoma, fibrosarcoma, chondrosarcoma, and malignant fibrohistiocytoma [9]. Management of FD ranges from observation to surgical intervention depending on patient symptomatology. Asymptomatic lesions can be observed for progression. Surgery is indicated when the patient has progressive deformity, large lesions with pain and loss of function, non-union, failure of nonsurgical therapy, or malignant transformation. Pain and deformity are signs that microfractures are developing in a lesion and should be addressed [6]. Although small focal lesions can be treated with cancellous bone grafts and curettage, local recurrence remains a problem. The process of creeping substitution, depending on the local healing response of the bone, involves resorption of the bone graft and newly formed host bone [10]. In FD, cancellous bone grafts undergo resorption and replacement with the same type of poorly formed woven bone and thus recurrence occurs, which may lead to fracture and deformity. Kokkalis et al. reported two cases of pathological fracture around elbow due to FD treated successfully with cancellous bone allograft after curettage. Both patients achieved excellent ROM and were pain free without any recurrences [11]. The use of cortical strut grafts for the management of FD lesions has been cited in literature [12, 13]. Vascularized autologous cortical bone does not weaken as it does not undergo resorption. It is remodeled in a fashion similar to normal bone and is a superior graft compared to its non-vascularized counterpart [12, 13]. One of the studies showed that non-vascularized fibular cortical strut autografts are a safe and effective method of treating benign lesions of proximal femur, including FD [14]. Kumta reported eight patients who were treated with vascularized bone grafting for the treatment of FD of the upper limb [15]. Through this case report, we aim to describe an innovative approach of managing fibrous dysplastic lesion of radius bone using non-vascularized fibular cortical strut graft. To the best of our knowledge, this has not been reported earlier, although, fibular cortical strut graft has been used to treat fibrous dysplastic lesion of proximal femur. We did not use any implant as we felt a tightly fitting cortical graft supported by external means would heal without displacement. Second, due to the short proximal fragment of the radius, no implant could provide any type of stability there. We admit that the distal end of the graft could have been stabilized by an intramedullary nail or a plate. Our technique thus avoided the use of any implant to fix the graft to the parent bone. It showed that a tightly fitted cortical graft is stable enough to incorporate with the original bone without deformation and pathological fracture, provided the forearm is protected till complete union is confirmed. No complication was observed postoperatively. The length of forearm along with the range of motion was maintained including supination and pronation of forearm. The hand and elbow function returned to the original pre-operative status.
Non-vascularized fibular cortical strut grafting is an effective treatment modality for FD of radius bone. External or internal fixation is not necessary if a tightly fitting cortical graft is jammed into the defect caused by lesion excision.
FD of radius bone is an uncommon presentation. If present involving more than half the length of radius bone, non-vascularized fibular cortical strut graft is a very effective modality for the same, where internal or external fixation is also not necessary. A tightly fitting graft jammed into the defect caused by lesion excision will suffice and there will be no need of any implant for fixation.
References
- 1.Lichenstein L. Polyostotic fibrous dysplasia. Arch Surg 1938;36:874-98. [Google Scholar]
- 2.Coley BL, Etkind I. Neoplasms of bone and related conditions: Etiology, pathogenesis, diagnosis, and treatment. Ann Surg 1962;155:471-9. [Google Scholar]
- 3.Benhamou J, Gensburger D, Messiaen C, Chapurlat R. Prognostic factors from an epidemiologic evaluation of fibrous dysplasia of bone in a modern cohort: The Francedys study. J Bone Miner Res 2016;31:2167-72. [Google Scholar]
- 4.Fitzpatrick KA, Taljanovic MS, Speer DP, Graham AR, Jacobson JA, Barnes GR, et al. Imaging findings of fibrous dysplasia with histopathologic and intraoperative correlation. Am J Roentgenol 2004;182:1389-98. [Google Scholar]
- 5.Moseley JN, Friedrich JB. Monostotic fibrous dysplasia of the distal radius metaphysis: Case report. Hand (NY) 2011;6:224-7. [Google Scholar]
- 6.Harris WH, Dudley HR, Barry RJ. The natural history of fibrous dysplasia: An orthopaedic, pathological, and roentgenographic study. J. Bone Joint Surg 1962;44:207-33. [Google Scholar]
- 7.Henry AK. Extensile Exposure. 2nd ed. New York: Churchill Livingstone; 1973. [Google Scholar]
- 8.Kransdorf MJ, Moser RP Jr., Gilkey FW. Fibrous dysplasia. Radiographics 1990;10:519-37. [Google Scholar]
- 9.Kushchayeva YS, Kushchayev SV, Glushko TY, Tella SH, Teytelboym OM, Collins MT, et al. Fibrous dysplasia for radiologists: Beyond ground glass bone matrix. Insights Imaging 2018;9:1035-56. [Google Scholar]
- 10.Burchardt H, Enneking WF. Transplantation of bone. Surg Clin North Am 1978;58:403-27. [Google Scholar]
- 11.Kokkalis ZT, Jain S, Sotereanos DG. Fibrous dysplasia around the elbow. J Shoulder Elbow Surg 2010;19:6-11. [Google Scholar]
- 12.Finkemeier CG. Bone-grafting and bone-graft substitutes. J Bone Joint Surg 2002;84:454-62. [Google Scholar]
- 13.Dell PC, Burchardt H, Glowczewskie FP Jr. A roentgenographic, biomechanical, and histological evaluation of vascularized and non-vascularized segmental fibular canine autografts. J Bone Joint Surg 1985;67:105-12. [Google Scholar]
- 14.George B, Abudu A, Grimer RJ, Carter SR, Tillman RM. The treatment of benign lesions of the proximal femur with non-vascularised autologous fibular strut grafts. J Bone Joint Surg 2008;90:648-51. [Google Scholar]
- 15.Kumta SM, Leung PC, Griffith JF, Kew J, Chow LT. Vascularised bone grafting for fibrous dysplasia of the upper limb. J Bone Joint Surg 2000;82:409-12. [Google Scholar]