Schwannoma with ancient change and cystic degeneration should be included in the surgeon’s differential diagnosis when a patient presents with intramuscular hematoma; therefore, biopsy is warranted since identification of a neoplastic process masquerading as a fluid collection drastically changes patient management.
Mr. Ryan J Giarraputo, Cooper Medical School of Rowan University, 401 South Broadway, Camden, New Jersey 08103. E-mail: giarra27@rowan.edu
Introduction: The value of this manuscript is that it highlights a common diagnostic challenge facing orthopedic surgeons, involving the reality that both benign and malignant soft-tissue tumors can present as large cystic masses masquerading as a hematoma. This is the first report of its kind to describe a schwannoma presenting as such a large hematoma in the thigh.
Case Presentation: A 64-year-old male presented with 2 days of worsening pain over a left posterior thigh mass that was enlarging for 12 years. Imaging demonstrated a cystic mass. 1.8L of serosanguinous fluid was aspirated and cytology was negative for malignancy, suggesting chronic hematoma. The fluid reaccumulated, indicating surgical management. Histopathology revealed a hemorrhagic ancient schwannoma
Conclusion: Without history of trauma or anticoagulation, intramuscular hematoma should be a diagnosis of exclusion. Burden of proof is high to rule-out a neoplastic process masquerading as fluid collection. Biopsies should be taken and schwannoma with ancient change and cystic degeneration should be considered.
Keywords: Hematoma, oncology, schwannoma
Schwannomas are benign neoplasms of Schwann cells that develop along peripheral nerves [1, 2, 3]. These tumors typically grow slowly, sometimes without symptoms [1, 2]. The majority of schwannomas occur spontaneously; however, they can arise in the setting of genetic diseases such as neurofibromatosis Type 1 and 2 (NF1 and NF2) [1]. A rare form of this tumor, known as an ancient schwannoma, is characterized histologically by major degenerative changes [2], including perivascular hyalinization, calcification, loss of hypercellular regions/nuclear degeneration, cystic necrosis, and hemorrhage leading to intralesional hematoma formation [2]. Akerman and Taylor were the first to describe this type of benign neurogenic tumor in 1951 as an “ancient neurilemmoma” [3] which account for 0.8% of all soft-tissue tumors [4]. On advanced imaging, schwannomas classically appear as well-defined, fusiform solid masses [5, 6] that are hypointense on T1 and hyperintense on T2, depending on the extent of fat content. Histologically, two cellular patterns are appreciated: Antoni A regions with Verocay bodies and Antoni B regions [2, 6]. Antoni A regions are hypercellular areas of spindle cells characterized by tightly arranged and elongated nuclei with central thickening and tapered ends [7]. Verocay bodies accompany these Antoni A regions and describe the clear areas between the sheets of spindle cells where cells do not have nuclei [7]. Antoni B regions are hypocellular with thin and wispy cells separated by microcystic spaces [2, 6, 8]. On immunohistochemical analysis, these stain positively for S-100 and SOX10 [1, 9]. When indicated, complete excision of the tumor should be performed to minimize the chance of recurrence [9, 10]. Hematomas are collections of bloody fluid within soft tissue. Histologically, hematomas show erythrocytes clumped together by fibrin strands along with pockets of serum. Typically hematomas self-resolve but they can be aspirated to facilitate culture and cytologic analysis if persistent [11]. If left untreated, hematomas can lead to tissue irritation, necrosis, and anemia due to blood loss [12]. This case study describes a rare presentation of a hemorrhagic ancient schwannoma masquerading as a massive hematoma. Despite several aggressive surgical debridements and attempts at preventing recurrence, the neoplastic nature of this lesion led to post-operative recurrent fluid collection. This report brings awareness to this rare entity, which is not traditionally associated with a blood-filled cystic space [2, 13], and aims to highlight that a hemorrhagic schwannoma should be considered in the setting of massive, recurrent, and atraumatic hematoma within muscle. Lee et al. [2] described two cases of an ancient schwannoma mimicking a malignant tumor, highlighting that after many years of chronic change, schwannomas can clinically and pathologically masquerade as other tumors. Our report is critical to the literature as it describes another entity that these ancient schwannomas can mimic, and surgeons must be aware of this fact to avoid misdiagnosis.
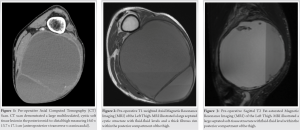
A 64-year-old male presented with 2 days of worsening left posterior thigh pain due to an enlarging mass. The patient stated that the mass had been slowly growing for 12 years, but only recently became painful. The patient was ambulating and denied trauma. He was not taking pharmacologic anticoagulation. On physical examination, the large left posterior thigh mass was firm, tense, and relatively non-tender to palpation. He exhibited full strength in all muscle groups, and full knee range of motion. Sensation was intact in all nerve distributions distally without paresthesias or numbness. Computed tomography and magnetic resonance imaging (MRI) demonstrated a large, multiloculated, and cystic mass in the posterior aspect of the thigh measuring 16.0 × 13.7 × 17.1 cm (Fig. 1, 2, 3). The mass abutted the posterior femoral cortex without cortical invasion and demonstrated splaying and edema of adjacent muscles. The sciatic nerve could be appreciated proximal to the mass and the tibial/common peroneal nerves could be visualized distal to it. However, at the level of the soft tissue mass, the nerve could not be visualized. Final radiology report suggested this to represent chronic hematoma.
Ultrasound-guided aspiration drained 1.8L of serosanguinous fluid from the mass. Cultures were negative. Cytology demonstrated presence of scattered lymphocytes, blood clot, hemosiderin-laden macrophages, and proteinaceous materials but was negative for neoplastic or atypical cells. Again, these findings were suggestive of chronic hematoma. Within 48 h, the fluid had recollected within the posterior thigh. A surgical irrigation and debridement was performed with open biopsy of the mass’s rind-like wall. A 9 cm longitudinal incision was made directly over the extremely large mass on the midline posterior thigh. The fascia was incised and approximately 500 mL of bloody fluid was immediately aspirated. A thick rind was present around the edge of the remaining deep fluid collection, which was incised using electrocauterization. On entering this deep space, another 1L of bloody fluid was aspirated. This entire space was cystic, filled with fluid and clot, with small areas of solid hypertrophic tissue at the periphery. The thick inner surface of the walls was bleeding in a capillary fashion, but the surgeon could not detect a large bleeding vessel.
Due to the surgeon’s suspicion of a vascular tumor, all of the clots and four areas of hypertrophic tissue from the inner rind were sent for pathologic examination. There was no obvious neural tissue within or surrounding the cavity, and a nerve stimulator was used to confirm this. The cavity was irrigated, and before washing, betadine and hydrogen peroxide were allowed to dwell for three minutes each. Electrocauterization was then used on all areas that were actively bleeding to achieve hemostasis. In fear of fluid reaccumulation, a vacuum-assisted closure device (VAC) was applied with plans for a future washout. Four days later, the patient returned to the operating room for VAC removal and repeat irrigation and debridement. A considerable amount of blood had recollected despite the VAC. Mechanical debridement of the inner walls of the cavity was again performed, including biopsy of two dilated and thrombosed vessels. Tisseel fibrin sealant (Baxter BioPharma Solutions, US) was then applied to all exposed surfaces in an attempt to control what appeared to be a diffuse neoplastic microvascular bleeding process. Two 15 French round JP drains were placed into the hematoma cavity. The lateral hamstring muscle belly was imbricated underneath the medial flap to obliterate dead space. Standard layered closure of the fascia, superficial tissue, and skin was performed. Sterile dressings were applied as well as a compressive wrap from toes to groin.
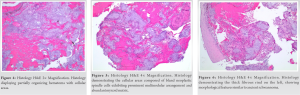
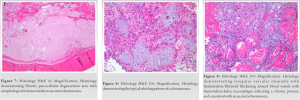
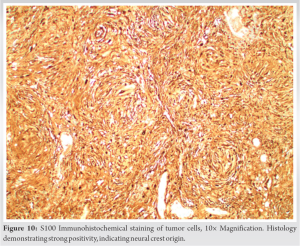
Final pathologic examination revealed that the samples primarily consisted of a partially organizing hematoma (Fig. 4) with cellular areas composed of bland neoplastic spindle cells exhibiting prominent multinodular arrangement and abundant myxoid matrix (Fig. 5). Fibrotic paucicellular areas that looked degenerative were also present (Figs. 6 and 7). Fig. 8 shows the typical whirling cellular pattern of a schwannoma. Irregular vascular channels with hyalinization/fibrinoid change and many hemosiderin-laden macrophages indicated a chronic process (Fig. 9). Immunohistochemical staining was strongly positive for S100 (Fig. 10) and SOX10, and negative for ERG, SMA, desmin, CD68, and MITF, consistent with peripheral nerve sheath tumor. Diagnosis of ancient schwannoma with secondary hemorrhage and hematoma formation was favored. Ten months postoperatively, the patient had recovered from surgery, could walk without assistance or pain, could perform daily activities, and had returned to work. He reported intermittent soreness at night in the left thigh and occasional sensory changes in the plantar left foot, relieved by gabapentin. Examination of the posterior left thigh demonstrated a visible and palpable collection about half its pre-operative size. Physical examination revealed full strength in all distal muscle groups and intact sensation.
Most schwannomas of the lower extremity present as nodular palpable masses with localized pain and positive Tinel’s sign [5]. The average size of schwannomas ranges between 2 and 20 cm, with a maximum of 5 cm in most extremities [5]. MRI shows the tumor as a smooth and well demarcated lesion which is isointense to muscle on T1 weighted images and homogeneously hyperintense on T2. Ancient schwannomas can traditionally be identified by their low-signal peripheral fibrous capsule surrounding the mass with internal T2 hyperintensity. In this way, ancient schwannomas can mimic chronic hematoma on MRI. Imaging that reveals a cystic structure with little solid component often leads to a shift in diagnostic focus away from neoplastic phenomena. However, both benign and malignant intratumoral hemorrhage can be difficult to distinguish from non-neoplastic hematoma [14]. History and pathologic examinations are critical in making this distinction. Nearly all intramuscular hematomas develop in the context of reported trauma and/or anticoagulation. In a case where neither trauma nor anticoagulation are present, a broad differential diagnosis should be explored. The surgeon should have a high index of suspicion that the fluid may originate from a hemorrhagic neoplasm [14, 15]. Several studies have described high-grade sarcomas masquerading as hematomas [14, 15] and reports exist of a posterior mediastinal schwannoma presenting with massive hemothorax in the absence of trauma [14]. Our case is consistent with these reports, and it highlights the high index of suspicion paired with histologic and immunohistochemical detection of neural tissue that ultimately led to the correct diagnosis.
Management of the degenerative ancient schwannoma with hematoma still needs to be explored. After two reaccumulations of the hematoma, use of cauterization and fibrin sealant followed by obliteration of dead space and multilayered closure minimized recurrence. Since the internal hemorrhage is a neoplastic phenomenon, it is the authors’ belief that reaccumulation of hematoma is more likely in these cases than in traditional non-neoplastic intramuscular hematomas. For this reason, more aggressive strategies must be employed and patient expectations must be managed. This can only occur if the surgeon is accurate in his/her understanding of the underlying pathology.
This diagnosis was challenging even for an orthopedic oncologist. It is necessary to train others on how to suspect malignant involvement.
References
- 1.Kavčič J, Božič M. Schwannoma of the tongue. BMJ Case Rep 2016;2016:bcr2016215799. [Google Scholar]
- 2.Lee YS, Kim JO, Park SE. Ancient schwannoma of the thigh mimicking a malignant tumour: A report of two cases, with emphasis on MRI findings. Br J Radiol 2010;83:e154-7. [Google Scholar]
- 3.Ackerman LV, Taylor FH. Neurogenous tumors within the thorax: A clinicopathological evaluation of forty-eight cases. Cancer 1951;4:669-91. [Google Scholar]
- 4.Dahl I. Ancient neurilemmoma (schwannoma). Acta Pathol Microbiol Scand Sect A 1977;85:812-8. [Google Scholar]
- 5.Maleux G, Brys P, Samson I, Sciot R, Baert AL. Giant schwannoma of the lower leg. Eur Radiol 1997;7:1031-4. [Google Scholar]
- 6.Wippold FJ 2nd, Lubner M, Perrin RJ, Lämmle M, Perry A. Neuropathology for the neuroradiologist: Antoni A and Antoni B tissue patterns. AJNR Am J Neuroradiol 2007;8:1633-8. [Google Scholar]
- 7.Wippold FJ 2nd, Lämmle M, Anatelli F, Lennerz J, Perry A. Neuropathology for the neuroradiologist: Palisades and pseudopalisades. AJNR Am J Neuroradiol 2006;27:2037-41. [Google Scholar]
- 8.McLendon RE, Bigner DD, Bigner SH. Intracranial and Intraspinal Schwannomas (WHO Grade I). Pathology of Tumors of the Central Nervous System: A Guide to Histologic Diagnosis. London: Arnold; 2000. p. 71-81. [Google Scholar]
- 9.Ishizaki T, Katsumata K, Enomoto M, Matsudo T, Shigoka M, Hisada M, et al. Successful laparoscopic resection of a retroperitoneal schwannoma arising from the femoral nerve: A case report. Asian J Endosc Surg 2017;10:194-7. [Google Scholar]
- 10.Guha D, Davidson B, Nadi M, Alotaibi NM, Fehlings MG, Gentili F, et al. Management of peripheral nerve sheath tumors: 17 years of experience at Toronto Western Hospital. J Neurosurg 2018;128:1226-34. [Google Scholar]
- 11.Elster A. Questions and Answers in MRI. MRI of Hemorrhage Overview. Available from: http://mriquestions.com/hematoma-overview.html#:~:text=The%20center%20of%20chronic%20hematomas,on%20T2*%2FSW%20images [Last accessed on 2021 Mar 09]. [Google Scholar]
- 12.Kaya G, Jacobs F, Prins C, Viero D, Kaya A, Saurat JH. Deep dissecting hematoma: An emerging severe complication of dermatoporosis. Arch Dermatol 2008;144:1303-8. [Google Scholar]
- 13.Ishibashi H, Takasaki C, Okubo K. Successful excision of a massive bleeding schwannoma by thoracoscopic surgery. Asian Cardiovasc Thorac Ann 2016;24:484-6. [Google Scholar]
- 14.Gomez P, Morcuende J. High-grade sarcomas mimicking traumatic intramuscular hematomas: A report of three cases. Iowa Orthop J 2004;24:106-10. [Google Scholar]
- 15.Taïeb S, Penel N, Vanseymortier L, Ceugnart L. Soft tissue sarcomas or intramuscular haematomas? Eur J Radiol 2009;72:44-9. [Google Scholar]