En bloc resection of distal radius with Brachytherapy gives favorable outcomes with less chances of recurrence.
Dr. Sagar Arora, Department of Orthopaedic Surgery, Guru Gobind Singh Medical College and Hospital, Faridkot - 151 203, Punjab, India. E-mail: sagar.arora504@gmail.com
Introduction: Giant cell tumors of the bone are aggressive and potentially malignant lesions. Juxtaarticular giant cell tumors of the lower end radius are common and is a challenge for reconstruction after tumor excision. Several reconstructive procedures like vascularized and non-vascularized fibular graft, osteoarticular allograft, ceramic prosthesis, and megapros thesis are in use for substitution of the defect in the distal radius following resection. Here, we have analyzed the results of aggressive benign Giant cell tumor of the distal radius treated by en bloc excision and reconstruction using autogenous non-vascularized fibular graft along with brachytherapy.
Material and Methods: Eleven patients with either Campanacci Grade II or III histologically proven giant cell tumors of lower end radius were treated with en bloc excision and reconstruction with ipsilateral non-vascularized proximal fibular autograft. Host graft junction was fixed with low contact dynamic compression plate (LC-DCP) in all cases. Fixation of the head of the fibula with carpal bones and distal end of the ulna, if not resected, using K-wires at graft host junction was done. Brachytherapy was given in all 11 cases. Routine radiographs and clinical assessments regarding pain, instability, recurrence, hand grip strength, and functional status were done using Mayo modified wrist score at regular intervals.
Results: The follow-up ranged from 12 to 15 months. At last follow-up, the average combined range of motion was 76.1%. The average union time was 19 weeks. Out of 11 patients, two patients had good results, five patients had fair results, and four patient had poor results. There was no case of graft fracture, metastasis, death, local recurrence, or significant donor site morbidity.
Conclusion: En bloc resection of giant cell tumors of the lower end radius is a widely accepted method. Reconstruction with non-vascularized fibular graft and internal fixation with LC-DCP along with brachytherapy minimizes the problem and gives satisfactory functional results with no recurrence.
Keywords: GCT, brachytherapy, en bloc resection, recurrence
Giant cell tumor (GCT) of bone is a benign but locally aggressive tumor with tendency for local recurrence, presenting in 3rd and 4th decade of life, and carries a definite female preponderance [1]. After distal femur and proximal tibia, distal radius happens to be the most common site of occurrence for GCT [1, 2]. Absence of absolute clinical, radiological, or histological parameters renders the tendency of any lesion to recur or metastasize. Treatment options for GCT at distal radius include curettage with bone grafting or cementing, en bloc excision and reconstruction with non-vascular or vascular fibular autograft, osteoarticular allograft, ulnar translocation, or endoprosthesis. Despite controversies, it is generally agreed that for a giant cell tumor of lower end radius, the extent of the surgical procedure and subsequent functional deficit must be weighed against the chance of recurrence [3]. Thorough curettage and bone grafting can preserve joint functions, it has been associated with high local recurrence rate of 27–54% [4, 5, 6, 7]. Walthar (1911) was the first to describe the use of a free non vascular proximal fibular graft to replace the resected distal radius [8]. In this prospective study of patients with giant cell tumor of the distal radius, we analyze the effectiveness of reconstruction of the defect after en bloc resection using non-vascularized fibular autograft along with brachytherapy.
Eleven patients with giant cell tumor of the bone at the distal end of the radius were treated by en bloc resection of tumor followed by reconstruction of the defect with autogenous non-vascularized fibular graft with a 12–15-month follow-up at our institution. There were four male and seven female patients. Their ages ranged from 25 to 50 years. The average follow-up was 13 months. Criterion for inclusion was evidence of radiographic features characteristic of GCT on roentgenogram, CT scan or MRI, and confirmation by histopathological examination. Campanacci’s staging system for giant cell tumor of the bone was used for staging. Grade I tumor had a well-marginated border of a thin rim of mature bone and the cortex was intact or slightly thinned but not deformed. Grade II tumor had relatively well-defined margins but no radio-opaque rim. Grade III tumors had fuzzy borders. According to this system, four were classified as Stage II and seven as Stage III.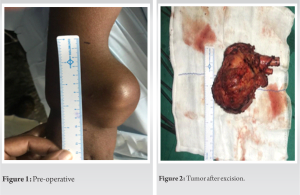
Procedure
Patients were operated under general anesthesia and ipsilateral forearm and leg were prepped and draped appropriately. A pneumatic tourniquet was used at both surgical sites. The tumor was approached through volar approach. Bone was resected at a level determined preoperatively based on extent of bone involvement on MRI plus a safe margin of 3–5 cm. On an average 10.5 cm (8–13 cm) of bone was resected. After excision, tumor bed was routinely treated with 5% phenol and 3% hydrogen peroxide to take care of the inadvertent spillage, if any. Ipsilateral fibula was approached from standard direct lateral approach after identifying and carefully protecting the common peroneal nerve. Fibula was sectioned at desired length depending on the defect created in forearm after tumor resection. We routinely obtained 3–5 mm extra length of fibula to cover for compression at radio fibular junction and error in taking measurements. The defect was bridged by the newly harvested non-vascularized proximal fibular autograft and was fixed using a 6 or 7 hole, 3.5 mm small fragment Low contact dynamic compression plate (LC-DCP). The proximal fibular graft was fixed with K-wire to the carpal bones and the distal ulna. In the same sitting, single plane implant was inserted with six needles with uniform spacing of 1.5 cm between each needle avoiding nerves and vessels if any. After careful hemostasis, wound was closed over a suction drain and an above elbow slab was applied. On post-operative day 2, computed tomography scan and planning was done, on day 3, brachytherapy was started in the dose of 4 Gy/Fraction with 6 h gap between fractions for 2 days (16Gy/4F/2Days). Brachytherapy was delivered by high dose rate nucleotron (microselectron) brachytherapy machine. No External beam radiotherapy was given in any case. Patients were followed-up at regular intervals and X-rays were taken at every visit. The aim of the early follow-up is to detect local recurrence. Clinical assessments regarding pain, instability, recurrence, hand grip strength, and functional status was done using the Mayo modified wrist score.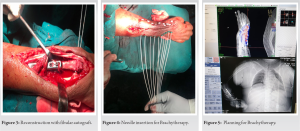

The present case series involved analysis of 11 patients with GCT lower end radius, four patient with Campanacci Grade 2 and seven patients with Grade 3. Of the 11 patients analyzed, there were four males and seven females with three left-sided and eight right sided involvement of distal radius. The mean age of patients included in analysis was 44.4 (25–50 years). The minimum age was 29 years and maximum age was 50 years. None of the cases had a pathological fracture or metastatic disease at presentation. Average size of the tumor resected in our study was 130.8 cc. Mean follow-up duration in our series was 13 months. Average time for radiological union was 19 weeks in our study. Brachytherapy was given in all 11 cases, irrespective of campanacci grading. Functional results were evaluated using the mayo modified wrist score. Out of 11 patients, two patients had good results, five patients had fair results, and four patients had poor results. One patient who was the known case of DM had Surgical site infection at 6-month follow-up and was treated appropriately with intravenous antibiotics and one patient suffered from non-union at the last follow-up. None of the patient had graft fracture, recurrence, or any sarcomatous transformation during the follow-up period.
GCT is a challenge for the orthopedic surgeons, both for cure and rehabilitation. Most patients with GCT are young with normal life expectancy. The aim of treatment is to excise the tumor, reduce the risk of recurrence, and preservation of the joint function. The problem of selecting proper treatment is complicated by the failure of its histologic appearance to indicate its biologic behavior [9]. Despite controversies, it is a general consensus that for a giant cell tumor of the lower end radius, the extent of the surgical procedure and subsequent functional deficit must be weighed against the chance of recurrence [3]. Eckardt et al. recommended en bloc resection for most grade III lesions [10]. Wide resection of the distal radius has been recommended to treat Grade III GCT when the tumor breaks through the cortex on dorsal and volar sides, when tumor invades the wrist joint or more than 50% of the surrounding metaphysis has been destroyed [11, 12]. Ipsilateral fibular non-vascularized autograft reconstruction of the large defect created after resection of tumor in lower radius offers many advantages over other procedures like more congruency of carpal joint, rapid incorporation as autograft, and easy accessibility without significant donor site morbidity. The present study was an attempt to analyze the outcomes of en bloc excision with defect reconstruction using autogenous non-vascularized fibular graft with addition of brachytherapy, primarily aiming to prevent the recurrence. The functional results obtained in our study were similar/to previously published series with average grip strength of 56.2% of contralateral normal side. Average time for union at host graft junction was 19 weeks in this series which is comparable to that reported by other authors where similar treatment option was used. Literature regarding the usage of vascularized fibula or prosthesis is found to be few and inconclusive [13, 14]. Vascularized fibular grafting has been reported to speed up the healing at host-graft junction thereby reducing the period of immobilization required. The operating time for vascularized fibular graft often reaches 12–14 h and requires sacrifice of two major vessels. Dissection to obtain the fibula and its vascular pedicle and the isolation of its recipient vessels requires meticulous attention, sophisticated infrastructure, skill, and prolonged operating time have made its use limited. Reconstruction over arthrodesis was preferred in our study to retain joint mobility. We preserved an average of 76.1% of the contralateral range of wrist motion. The grip strength compared to the contralateral hand was found to be 56.22%. Many authors reported good results (score around 74%) with arthrodesis for distal radius GCT [15, 16] Wrist arthrodesis with autogenous fibula or ulna has been used after resection. An arthrodesis produces a painless and stable wrist, though at the expense of mobility. An arthrodesis reduces the functional score with minimal disability [17] Partial wrist arthrodesis has advantages over total arthrodesis [18]. Local recurrence of GCT is a very common problem reported in the literature. Zou et al. reported 25.9% recurrence rate of GCT in radius, out of which recurrence rate in curettage group was 27% compared to en bloc resection group in which recurrence rate was 23.8% [19]. In our study, no local recurrence was reported both clinically and radiologically supporting the use of brachytherapy along with current management procedures for the GCT distal radius. Many authors in the literature have reported many complications following en bloc excision such as carpal subluxation, infection, and graft fracture resulting in poor functional outcomes. In our study, one patient reported SSI at 6-month follow-up which was treated appropriately with prolonged course of intravenous antibiotics. One patient who was the known case of DM from the past 20 years developed non-union and was given the option of bone grafting but the patient refused for any further surgical intervention. None of the patient had graft fracture or carpal subluxation. Another possible complication or risk reported in the literature with the use of brachytherapy is malignant or sarcomatous transformation of GCT. Palmerini et al. analyzed that the cumulative incidence of malignancy of GCT was 4.0% out of which the cumulative incidence of primary malignancy was 1.6% compared with 2.4% for secondary malignancy. They confirmed that most malignant GCT of bone is secondary and occurs following radiation [20]. In our series, no episode of malignant transformation was reported during the follow-up period suggesting the use of brachytherapy in the above mentioned dosage for the treatment of GCT lower end radius. The present study had a limitation of small sample size in a single center but a large randomized, controlled, and multi-center trial would be impractical considering the incidence of such tumors. Nevertheless, the conclusions should be further confirmed by a large prospective study with a longer follow-up.
En bloc resection of giant cell tumors of the lower end radius followed with reconstruction of the defect with non-vascularized fibular graft, internal fixation with LC-DCP supplemented with brachytherapy gives satisfactory functional results with no local recurrence.
Treating GCT of distal radius is a challenging task. Best treatment option available along with other treatment option should be discussed with the patient for the satisfactory functional outcome. Hence, our study might provide a potential guiding light for the addition of brachytherapy in the management principles of GCT in distal end radius in the future.
References
- 1.Unni KK, Inwards CY. Dahlin’s Bone Tumors: General Aspects and Data on 10,165 Cases. Philadelphia, PA: Lippincott Williams and Wilkins; 2010. p. 225-42. [Google Scholar]
- 2.Goldenberg RR, Campbell CJ, Bonfiglio M. Giant-cell tumor of bone. An analysis of two hundred and eighteen cases. J Bone Joint Surg Am 1970;52:619-64. [Google Scholar]
- 3.Lackman RD, McDonald DJ, Beckenbaugh RD, Sim FH. Fibular reconstruction for giant cell tumour of the radius. Clin Orthop Relat Res 1987;218:232-8. [Google Scholar]
- 4.Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumour of bone. J Bone Joint Surg Am 1987;69. [Google Scholar]
- 5.Getilis S, Mallin BA, Piaseccki P, Turner F. Intralesional excision compared with en bloc resection for giant cell tumour of bone. J Bone Joint Surg Am 1993;75:1648-55. [Google Scholar]
- 6.McDonald DJ, Sim FH, McLeod RA, Dahlin DC. Giant cell tumour of bone. J Bone Joint Surg Am 1986;68:235-42. [Google Scholar]
- 7.Griend RA, Funderburk CH. The treatment of giant cell tumour of distal part of the radius. J Bone Joint Surg Am 1993;75:899-908. [Google Scholar]
- 8.Walthar M. Resection de extremeteinferieure du radius pour osteosarcoma geffe de I extremitesuperiuete du perone. Sac Chir Par Bull Mem 1911;37:739-47. [Google Scholar]
- 9.Smith RJ, Mankin HJ. Allograft replacement of distal radius for giant cell tumor. J Hand Surg Am 1977;2:299-308. [Google Scholar]
- 10.Eckardt JJ, Grogan TJ. Giant cell tumour of bone. Clin Orthop Relat Res 1986;204:45-58. [Google Scholar]
- 11.Cheng CY, Shih HN, Hsu KY, Hsu RW. Treatment of giant cell tumour of the distal radius. Clin Orthop Relat Res 2001;383:221-8. [Google Scholar]
- 12.Harness NG, Mankin HJ. Giant cell tumour of the distal forearm. J Hand Surg Am 2004;29:188-93. [Google Scholar]
- 13.Pho RW. Malignant giant cell tumour of distal end of the radius treated by a free vascularized fibular transplant. J Bone Joint Surg Am 1981;63:877-84. [Google Scholar]
- 14.Ihara K, Doi K, Sakai K, Yamamoto M, Kanchiku T, Kawai S. Vascularized fibular graft after excision of giant cell tumour of the distal radius. A case report. J Surg Oncol 1998;68:100-3. [Google Scholar]
- 15.Murray JA, Schlafly B. Giant cell tumours in the distal end of the radius. Treatment by resection and autograft interpositional arthrodesis. J Bone Joint Surg Am 1986;68:687-94. [Google Scholar]
- 16.Panchwagh Y, Puri A, Agarwal M, Anchan C, Shah M. Giant cell tumour-distal end radius: Do we know the answer. Indian J Orthop 2007;41:139-45. [Google Scholar]
- 17.Puloski SK, Griffin A, Fergusson PC, Bell RS, Wunder JS. Functional outcome after treatment of aggressive tumours in the distal radius. Clin Orthop Relat Res 2007;459:154-60. [Google Scholar]
- 18.Minami A, Kato H, Iwasaki N. Vascularized fibular graft after excision of giant cell tumour of the distal radius: Wrist arthroplasty versus partial wrist arthrodesis. Plast Reconstr Surg 2002;110:112-7. [Google Scholar]
- 19.Zou C, Lin T, Wang B, Zhao Z, Li B, Xie X, et al. Managements of giant cell tumor within the distal radius: A retrospective study of 58 cases from a single center. J Bone Oncol 2019;14:100211. [Google Scholar]
- 20.Palmerini E, Picci P, Reichardt P, Downey G. Malignancy in giant cell tumor of bone: A review of the literature. Technol Cancer Res Treat 2019;18:1533033819840000. [Google Scholar]











